How To: Drawing Lewis Structures From Condensed Molecular Formulas
Drawing
Lewis structures from condensed structural formulas is a survival skill for
organic chemistry students. There
are three steps you should follow to draw a correct structure.
1.
From a condensed
molecular formula, you obtain information about which atoms are connected to
each other in a molecule. Connect
all of the appropriate atoms with single bonds first (lines).
Example:
CH3CH2CH2CO2CH3
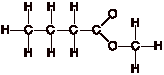 Comment: The difficult part of this
structure is deciding how to arrange the two oxygen atoms. Using the
arrangement shown will produce a stable structure with filled valences for all
of the atoms upon completion of step 3. With practice you will begin to
recognize common functional groups such as the carboxylic ester group of this
example. If you are unsure, you must draw the different possibilities you are
considering, and following completion of the structure, determine which one
produces the stable structure with the maximum number of filled valence shells
around the atoms.
Comment: The difficult part of this
structure is deciding how to arrange the two oxygen atoms. Using the
arrangement shown will produce a stable structure with filled valences for all
of the atoms upon completion of step 3. With practice you will begin to
recognize common functional groups such as the carboxylic ester group of this
example. If you are unsure, you must draw the different possibilities you are
considering, and following completion of the structure, determine which one
produces the stable structure with the maximum number of filled valence shells
around the atoms.
2.
Add all of the
additional valence electrons for each atom that does not already have a filled
valence shell due to the single bonds.
Remember that for the purpose of counting valence electrons around
atoms, assign one electron to each atom taking part in a single bond. Make sure to keep track of any formal
charges that may be present in the condensed structural formula (the present
example has none).
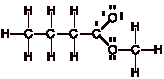 Comment:
Recall that each neutral carbon atom has 4 valence electrons, and each neutral
oxygen atom has 6 valence electrons. After taking into account all of the
single bonds in the molecule, the carbon atom connected to both oxygen atoms has a single
electron left over (4 total electrons - 3 single bonds = 1 electron left over),
the oxygen atom attached only to one carbon atom has five electrons left over
(6 total electrons Ð 1 single bond = 5 electrons left over) and the other
oxygen atom has four electrons left over (6 total electrons Ð 2 single bonds =
4 electrons left over).
Comment:
Recall that each neutral carbon atom has 4 valence electrons, and each neutral
oxygen atom has 6 valence electrons. After taking into account all of the
single bonds in the molecule, the carbon atom connected to both oxygen atoms has a single
electron left over (4 total electrons - 3 single bonds = 1 electron left over),
the oxygen atom attached only to one carbon atom has five electrons left over
(6 total electrons Ð 1 single bond = 5 electrons left over) and the other
oxygen atom has four electrons left over (6 total electrons Ð 2 single bonds =
4 electrons left over).
3.
Add multiple bonds
to eliminate unpaired electrons.
Draw remaining non-bonding electrons as lone pairs.
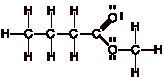
Comment: The only unpaired electrons were on carbon and oxygen,
leading to one new bond being formed.
The Lewis structure is now complete. The good news
is that this will get easier. After you practice so that you are comfortable
drawing Lewis structures by counting valence electrons, you will begin to
recognize functional groups based on the numbers of bonds and lone pairs on the
atoms. For example in molecules with no formal charges (Section 1.2E), hydrogen
has one bond and no lone pairs, carbon has four bonds and no lone pairs,
nitrogen has three bonds and one lone pair, oxygen has two bonds and two lone
pairs, and halogens have one bond and three lone pairs. Remember that double
bonds count as two bonds, and triple bonds count as three bonds in this type of
analysis.
Atoms with formal charges can also be recognized
by their characteristic number of bonds and lone pairs. Positively charged nitrogen has four
bonds, oxygen three bonds and one lone pair, and carbon three bonds (unfilled
valence shell). Negatively charged nitrogen has two bonds and two lone pairs,
oxygen one bond and three lone pairs, and carbon three bonds and one lone pair.