STEREOCHEMISTRY
HOPEFULLY MADE SIMPLER
Stereoisomers-molecules that have the same constitution, but different disposition of
groups in space. In other words
the atoms are connected to each other in the same way, they only differ with
respect to relative orientation in three dimensional space.
Chiral-General Definition-Any object that is not superimposable on its mirror
image. Your hands are chiral, that
is why you need two different leather gloves, one that only fits your right
hand, and one that only fits your left hand. If your hands were superimposable, then you would only need
one kind of glove and it would fit both hands.
Chemistry
Definition; Atom-Any tetrahedral
carbon atom that has four different substituents is a stereocenter. Any tetrahedral carbon atom that has
four different substituents is a stereocenter (it was worth repeating).
This is a simple consequence of geometry; there are two different ways to place four different substituents in a tetrahedral
arrangement. Looking for
four different substituents on a single carbon atom is the easiest way to
identify a chiral molecule.
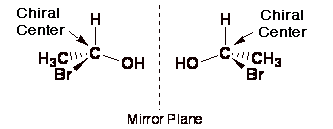
A
carbon atom is not a
stereocenter if even two of the substituents are the same. A carbon atom is not a stereocenter if even two of the substituents are the
same (it was worth repeating).
Historical note: in the past, stereocenters have also been called;
stereogenic centers, asymmetric carbon atoms, asymmetric centers, chiral
centers, or chiral atoms even though these latter names can be confusing in certain
situations.
Chemistry
Definition; Molecule- A molecule is
not chiral (even if it has stereocenters) if it has a plane or center of
symmetry. A plane of symmetry is any plane cutting through the molecule such that
one side is a perfect reflection of the other. When looking for a plane of symmetry you have to
put the molecule in the most symmetric conformation possible, DO NOT WORRY
ABOUT WHICH CONFORMATION IS MOST STABLE, STABILITY OF CONFORMATION IS NOT
IMPORTANT FOR STEREOCHEMISTRY. This means you look for symmetry in
cyclohexane derivatives that are drawn flat and alkanes that are eclipsed !
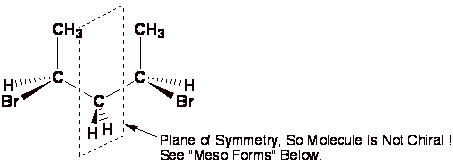
A center of symmetry is any point is space such that any group on the
molecule can be reflected back through that point an equal distance but
opposite direction and an equivalent group is found. This is relatively rare in organic chemistry.
As
a further consequence of simple geometry, there are only two different ways to
place four substituents around a stereocenter, and as shown above the resulting
two different molecules are mirror images of each other. They are non-superimposable mirror
images of each other. You should
make models of the above molecules and prove to yourself they are different and
non-superimposable if you haven't done this already. This pair of chiral molecules that are mirror images of each
other are called enantiomers,
a chemistry name given to represent this special mirror image relationship
between molecules.
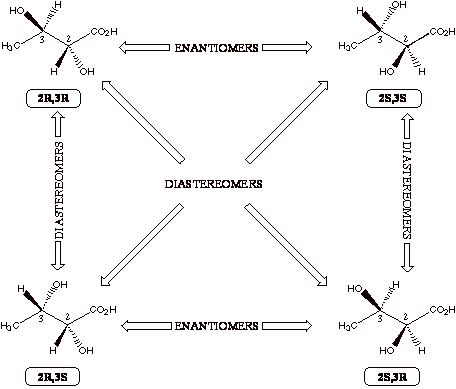
Molecules With More Than One Chiral Center If
there are two stereocenters in a single molecule, there are four possible
stereoisomers. This is because
each carbon atom can be in one of two possible forms (R or S) as we saw above,
so there are 2x2=4 possible combinations.
Now refer to the general figure below as well as the figure on the
following page with the four chemical structures. Make sure you understand all the relationships of the four
different molecules shown. It is easiest
to remember that enantiomers must be mirror images of each other. Diastereomers is the chemistry term that describes the
relationship between the pairs of molecules in the figures that are not
even mirror images of each other.
In other words, each pair of molecules in the figures must be related
because they are all stereoisomers of each other, so they are either
enantiomers (mirror images) or diastereomers (not mirror images). Thus, diastereomers are
stereoisomers that are not enantiomers. If a molecule happens to
be symmetric so that two of the four possible stereoisomers are identical
(the S,R is identical to the R,S; the S,S and R,R will always be
enantiomers), this form of the molecule is called the meso form. Since
this situation requires some special symmetry (usually a plane of symmetry) to
be present, it is the rare exception, not the rule.
To Determine Whether Molecules Are Enantiomers,
Diastereomers or Meso Compounds On A Test: *
1). Determine
the absolute (R or S) stereochemistry at each chiral atom.
2). Use
the following table to determine relationship.
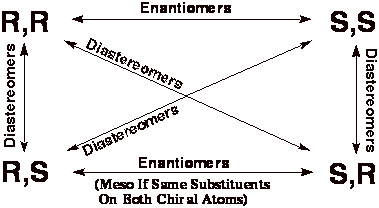
3). If
the molecule happens to be symmetric, check for meso compound, remembering that
only the R,S or S,R molecules can be meso (the S,S and R,R forms of even symmetric
molecules are not meso compounds, they are enantiomers).
* This method is much easier than simply trying to
look for mirror images etc. by rotating molecule in your mind or redrawing
structures in various orientations.
Other Important Ideas:
1). Different
enantiomers can only be distinguished by chiral things (optical activity etc.).
Living systems and biological molecules are chiral,
so chiral molecules are almost always distinguished by living systems.
2). Different
diastereomers can be distinguished by all of their chemical properties.
3). A racemic
mixture is defined as being a
one-to-one mixture of two enantiomers.