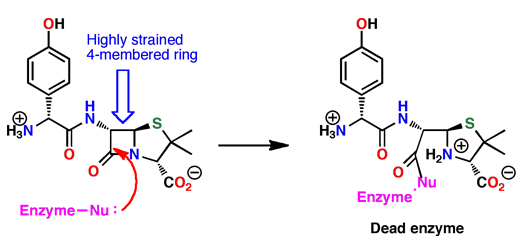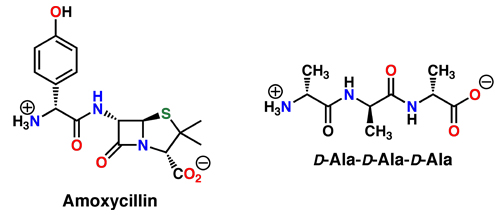
The very unusual four-membered ring of the penicillins is called a beta-lactam. This ring has a great deal of ring strain in it, so it can be attacked by nucleophiles inside the bacterial enzymes to open the ring as shown below. However, the new bond created with the enzyme is permanent, because reversing the reaction would involve making the strained ring, and this is too unfavorable. The enzyme is covalently linked to the penicillin, and therefore permanently shut down. In other words, the ring strain in the beta-lactam ring is released by reacting with the enzyme, giving such a strong "motive" that the process is essentially irreversible. The bacteria cannot make strong cells walls and they start dying.