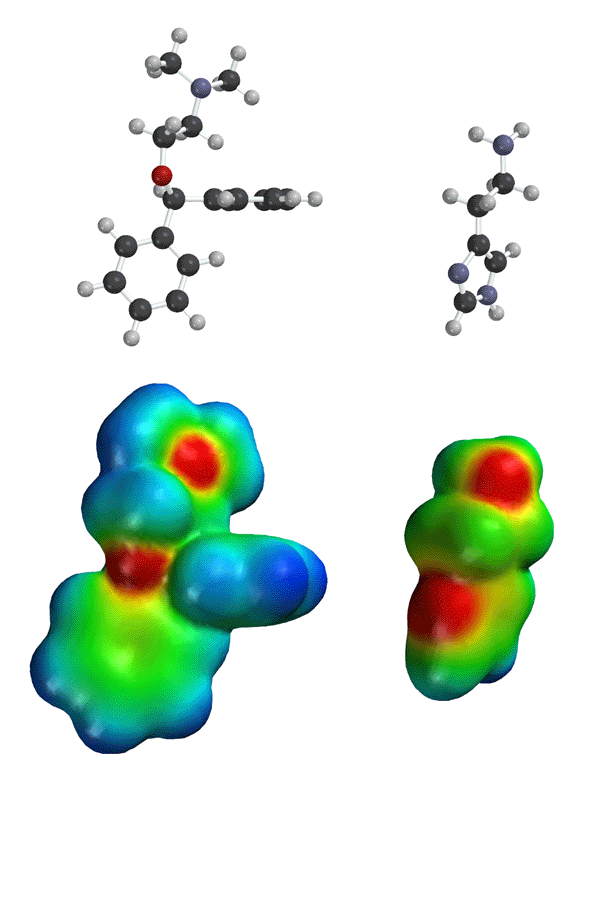|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecule
of the Day: Benadryl |
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
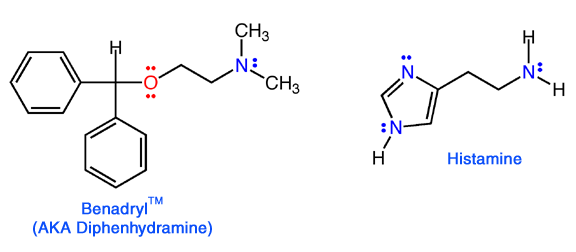
Histamine
is used a signal in the body. Duing an allergic response, in your nose for
example, if a pollen grain is recognized by specific receptors that trigger
release of histamine. (In hayfever, pollen is mistaken for a parasite by
allergic individuals.) The histamine, in turn, causes the release of mucous
to try and eliminate the invading parasite/pollen. |
|
|
|
|
|
|
|
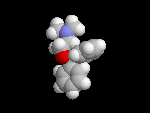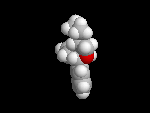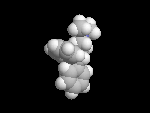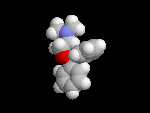
Benadryl
is classified as an antihistamine, because it decreases the amount of histamine
released when a person is exposed to an allergen. The
question is: why does it act as an antihistamine when at first glance it
does not look that much like histamine? |
|
|
|
|
|
|
|
|
|
Mode of action. Benadryl is mistaken for histamine in
the body, but benadryl does not cause mucous release. Rather, it just
binds where histamine ordinarily would, preventing histamine from triggering
the release. This mode of action is referred to as being an "antagonist".
The question remains, why does the body mistake benadryl for histamine
when they look so different? The answer, as always, can be found be examining
where the electrons are in the two molecules......
|
|
|
|
|
|
|
|
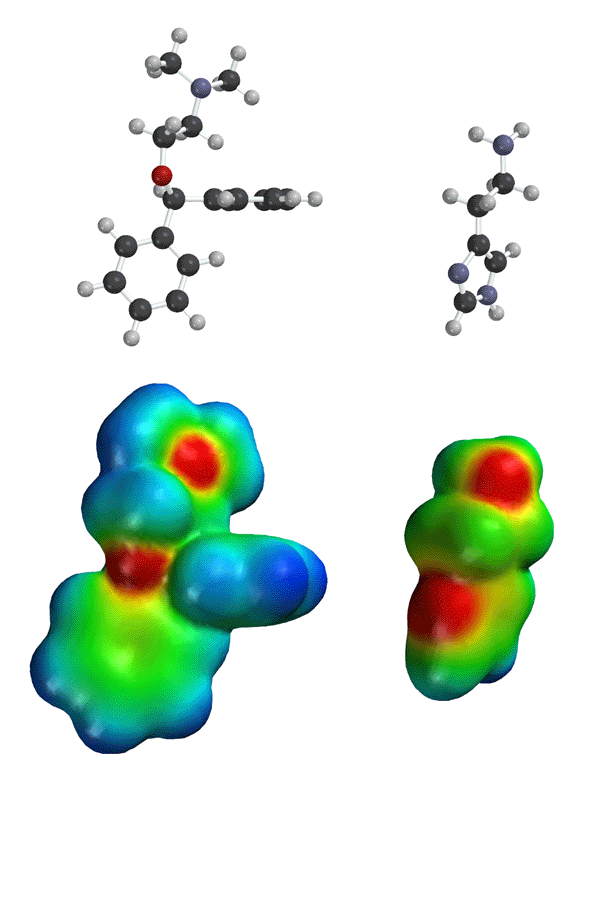 |
|
|
|
|
|
As can
be seen on the above electrostatic potential surfaces, both benadryl and
histamine have areas of high electron density (red color) in the same areas
of three-dimensional space. Thus, it is now easier to see how benadryl could
be mistaken for histamine in the body! This area of benadryl that is similar
in structure and electron density distribution to the natural molecule (histamine)
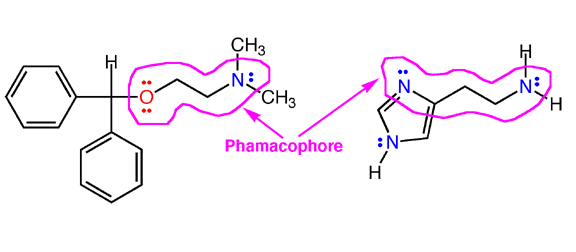
is referred to as a "pharmacophore".
This is the key concept in modern pharnaceutical science, and helps explain
how drugs work in the body. Similar stuctures and electron density distributions
between a natural molecule and drug. Note, benadryl has one side effect
of making you drowsy, because it crosses the blood-brain barrier and interacts
with molecules there that regulate alertness. Drugs often have side effects
becuase they interact at pars of the body not intended. |
|
|
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |