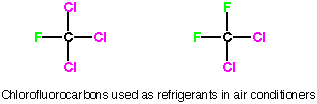
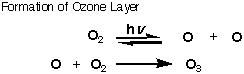
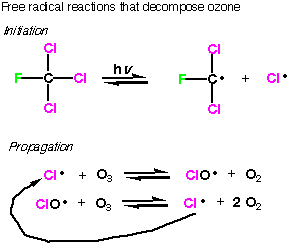
The true effects are still being studied and the exact amount of damage caused by CFC’s are still being debated. The ozone hole over the Antarctic is real and its fluctuations are being analyzed every year. It is important to take the safe approach, and CFC’s are being phased out all over the world. They are being replaced by similar molecules that have been chosen because they do not dissociate into chlorine radicals. In the replacements, thre are still fluorine and chloring atoms, but the chlorine and fluorine atoms are attached to different carbon atoms. This dramatically decreases the stability of the carbon radical (the attached fluorine atoms stabilize the radical formed from first generation CFC's, which may be counterintuitive to you at this point, but trust me) so the propagation steps are no longer favored and the chain reaction does not start. Hydrofluorocarbons (HFA's), that is refregerants with no chlorine, are also being used as CFC replacements.
Check how the ozone layer is doing on this official NASA page.
Abstract of general article on CFC's and ozone layer depletion
Abstract of a nice article on skin cancer and repair mechanisms induced by UV light exposure.