Hydrogen Bonding in Drug-Receptor Interactions
Hydrogen bonds have directionality in that the donor and acceptor groups must
be oriented appropriately with respect to each other for a hydrogen bond to
form. Important hydrogen bond donor groups in biological molecules include
the –OH groups of proteins and carbohydrates. Important hydrogen bond
acceptor groups are any N or O atom with a lone pair such the oxygen atom
of a carbonyl group (proteins, nucleic acids) and the oxygen atom of a hydroxyl
group (proteins and carbohydrates).
With directionality comes the potential for hydrogen bonds to organize molecules
at many levels ranging from the specific folding of biological molecules to
the specific binding and recognition between a pharmaceutical and its receptor.
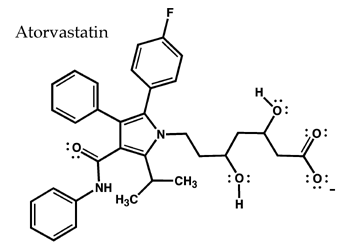
The drug atorvastatin (Lipitor) is used to treat high cholesterol. Cholesterol
is synthesized in the liver from the two-carbon acetyl group of a molecule
called acetyl coenzyme A (acetyl-CoA). A key intermediate in the sequence
of reactions leading to the synthesis of cholesterol is a six-carbon molecule
named mevalonate (Section 26.4B). Atorvastatin specifically binds to, and
blocks, the action of HMG-CoA reductase, a key enzyme required for the biosynthesis
of mevalonate. Atorvastatin binds to this enzyme in preference to the large
number of other potential enzyme targets because (1) this drug has a shape
that is exactly complementary to the catalytic cavity of HMG-CoA reductase,
and (2) it can form at least nine specific hydrogen bonds with functional
groups at this site on the enzyme. The complementary shape and pattern of
hydrogen bonding insure that atorvastatin binds to HMG-CoA reductase and inhibits
its ability to catalyze the formation of mevalonate. The hallmark of this
and other effective drugs is their ability to bind strongly with their intended
targets, while avoiding interactions with other proteins that could lead to
unwanted side effects.
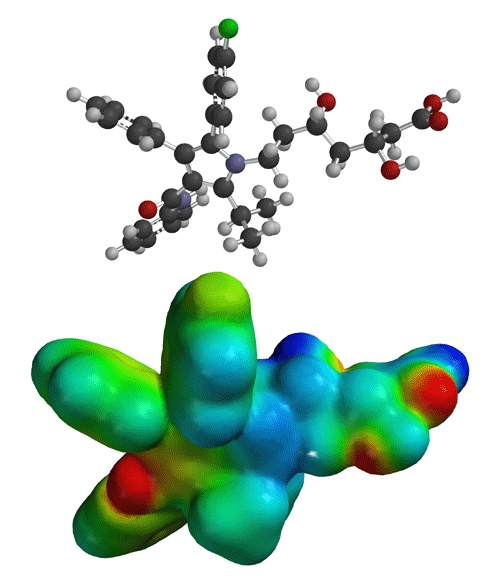
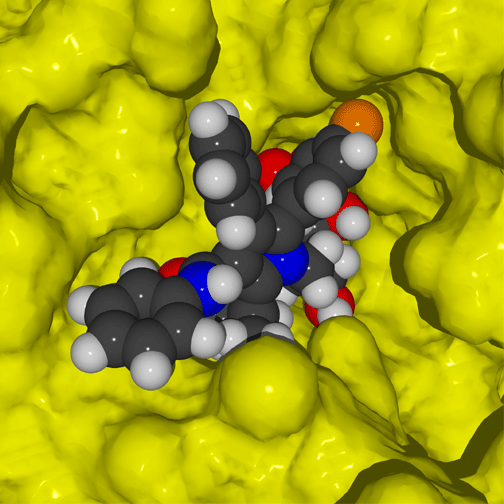
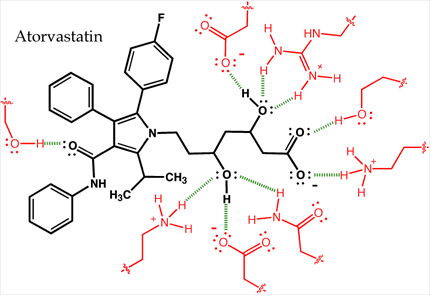
Hydrogen bonding between the cholesterol-lowering drug atorvastatin and the enzyme HMG-CoA reductase (shown in red). These nine hydrogen bonds, many of which involve hydroxyl groups on atorvastatin or the enzyme, provide specificity.
Click here for a general review article on atorvastatin and how it can reduce the risk of heart disease or stroke.
Click here for a general review of the history of atorvastatin and how it was developed.