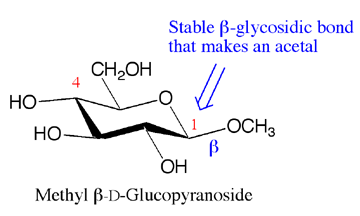
Carbohydrate monomers such as D-glucose, D-fructose or D-galactose are called monosaccharides. They are cyclic hemiacetals. Carbohydrates can be turned into cyclic acetals by reaction with an alcohol such as methanol in acid. The reaction follows the same mechanism as regular acetal formation. The bond to the alcohol is given the special name of glycosidic bond. If it is made from the alpha anomer, it is called an apha-glycosidic bond. If it is made from the beta anomer it is called a beta glycosidic bond.
Turning a monosaccharide into an acetal has an important consequence. The anomers do not interconvert without strong acid so the alpha anomer will stay alpha and the beta anomer will stay beta. Shown below is methyl beta-D-glucopyranose, the methyl acetal of glucose with a beta glycosidic bond.
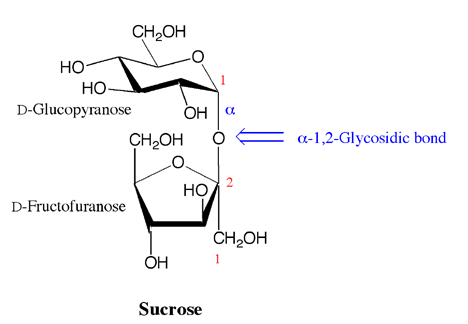
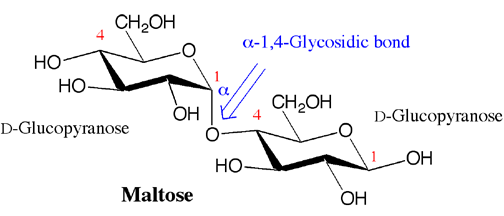
Specific enzymes can put carbohydrates together by making glycosidic bonds between the anomeric carbon of one carbohydrate and one of the -OH groups on another carbohydrate. The result is a molecule with two saccharides so they are called disaccharides. Note that the glycosidic bond can be alpha or beta, and that the second carbohydrate can be linked at any of the carbon atoms that contain an -OH. The glycosidic bond is named as alpha or beta, followed by numbers that correspond to the locations of the carbons involved in the glycosidic bond. Importantly, as described above, the glycosidic bond that is formed prevents alpha beta interconversion at the acetal carbon, so an alpha linkage remains an alpha linkage and a beta linkage remains a beta linkage.
Many important carbohydrates in biochemistry are really disaccharides. For example, maltose, the form of sugar found in malted grain, is really a disaccharide with an alpha-1,4-gylcosidic bond between carbons 1 (the anomeric carbon) and 4 of two D-glucopyranose molecules (i.e.D-glucose in the cyclic form).
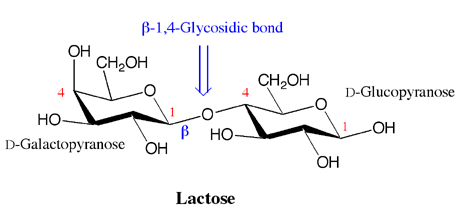
Lactose, the carbohydrate that is most common in milk has a beta-1,4 glycosidic bond between carbon 1 of D-galactopyranose (D-galactose) and carbon 4 of D-glucopyranose (D-glucose). Individuals who are lactose intolerant lack the digestive enzyme required to break this specific glycosidic bond. Bacteria in the digestive system use the lactose as a food source, leading to the symptoms of lactose intollerant people. The cure is ingesting some of the enzyme prior to eating or drinking milk products.