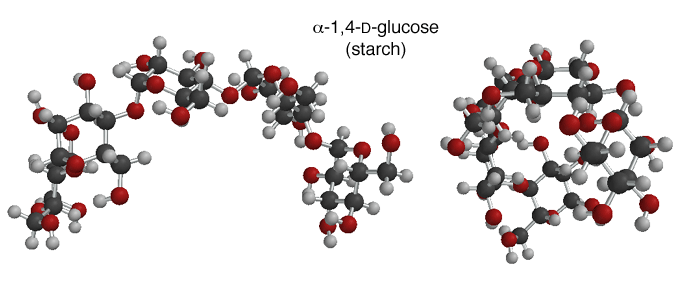
Carbohydrate monomers such as D-glucose are converted into polymers for various pursposes. The key features of these polymers are 1)the configuration of the glucosidic bonds between D-glucose units and 2) the location of the links. For the two most important D-glucose polymers, the linkage is between carbons 1 and 4. The linkage can be alpha (axial) or beta (equatorial). The configuration of the glycosidic linkage has profound significance to the properties of the polymer.
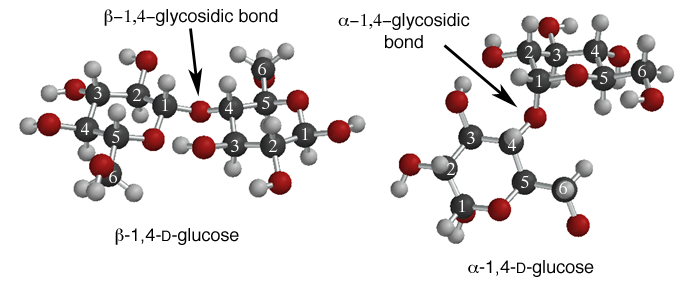
If the linkage is beta, the entire structure can be planar. The OH groups on the individual glucose units can take part in hydrogen bonds between different polymer chains, and the entire structure can make a planar assembly, that is very strong. Individual planes stack on top of each other to make a very strong three dimensional structure. This is actually cellulose, a polymer of beta-1,4-D-glucose that is the major component of wood!! The important point is that is all starts with the configuration of the glycosidic bond. Because the beta linkage allows a planar structure, the entire polymer is planar and can assemble into a very strong three dimensional material.
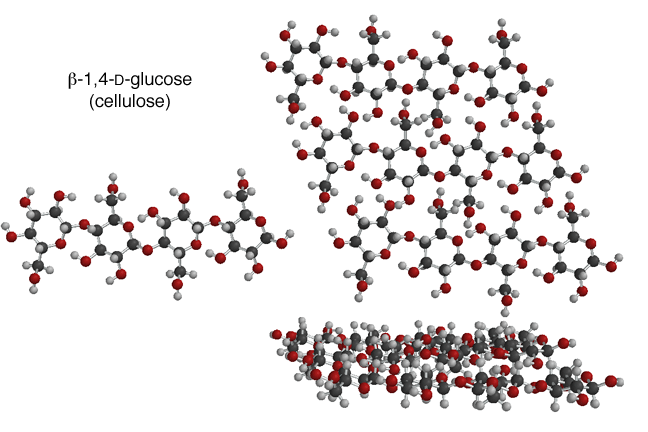
The alpha-1,4 glycosidic bond puts a "bend" in the polymer chain, so alpha-1,4-D-glucose units in a polymer chain has very different properties. This polymer is made of chains that cannot assemble into a strong structure so it makes a soft material. Cell store excess glucose as polymers with mostly alpha-1,4-glycosidic bonds, but also some alpha-1,6-glycosidic bonds. This material is the starch, as in potatoes, and glycogen in mammals. When you are "training" in distance athletic events, part of the process is building up glycogen stores that can be called upon when you need extra glucose. "Hitting the wall" refers to when your body runs out of stored glycogen and must activate other biochemical processes to obtain glucose to fuel your excercise. The important point is that the difference between a potato and wood is the configuration of the glycosidic bond, and really whether the bond makes a bend or not.Once again, molecular structure leads to predictable chemical properties! Remember this next time you are eating a French fry on a wooden table!!