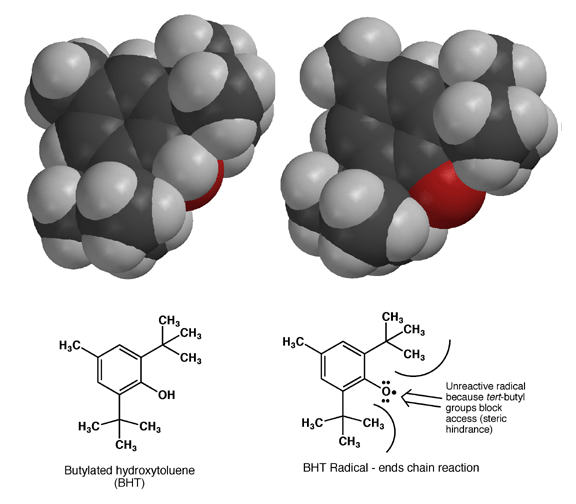
Once formed, the BHT radical cannot react further because the large tert-butyl groups create so much steric hindrance that the oxygen atom bearing the unpaired electron density cannot make the required contact with other molecules to allow a reactions. In this way, formation of the BHT radical stops radical chain reactions. This is not an accident, BHT was designed to make a very stable radical due to resonance that would not react further due to steric hindrance. Aren't chemists clever?!
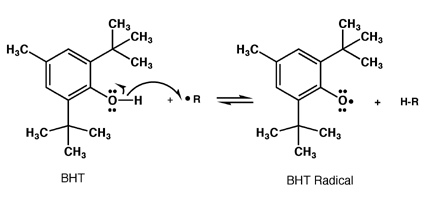
BHT added to food will react with any radicals formed to create the BHT radical,
which cannot react further. In this way, radical chain processes that attack
fatty acids are circumvented and food does not spoil as quickly. The irony
is that food purchased without preservatives due to fears of the danger of
having additives may spoil sooner and present a greater health risk than the
preservative.
Although preservatives have periodically received negative media attention,
it turns out that peroxides and peroxyradicals in spoiling food are likely
far more dangerous than the BHT could ever be. Peroxides and peroxyradicals
are known carcinogens. In fact, vitamin E works in almost the exact same way
to break radical chain reactions inside our bodies, and taking does of vitamin
E is thought by some to be important for preventing free radicals in our bodies.
By the way, worrying about radicals in our bodies is not far fetched, as free
radicals, especially those associated with energy production in our mitochodria
and immune system cells have been at least implicated in a number of disease
states and confirmed in others. Large numbers of studies have so far found
little danger associated with moderate amounts of preservatives such as BHT,
and BHT has even been found to prevent some disease states when given to patients
as an anti-oxidant.