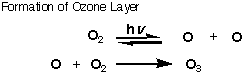|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecule
of the Day: CFC's |
|
|
|
|
|
|
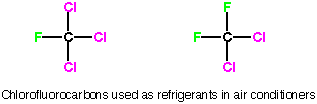
Chlorofluorocarbons
(CFC’s) have been used for a long time as refrigerants because they have
the proper boiling point to operate in condense (high pressure)-evaporation
(low pressure) cycles inside refrigerators and air conditioners. When they are
under pressure they are a liquid. When they are put into a chamber with less
pressure, they evaporate, which causes cooling. This cycle is repeated as necessary. |
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
The
problem is that CFC’s are gasses and they diffuse up into the upper reaches
of the atmosphere. Ozone (O3) is a poisonous gas. However,
at 12-15 miles up in the atmosphere it is a lifesaver. Ozone is formed when
oxygen (O2) is exposed to intense ultraviolet rays coming
from the sun. Once formed, the ozone (O3) absorbs the
harmful ultraviolet rays that would damage living creatures, especially us.
Some harmful ultraviolet rays get through, and among other things, it leads
to mistakes in DNA that can cause cancer, especially skin cancer. Greater lifetime
skin exposure means a greater lifetime chance of skin cancer, and severe sunburns
when young are particularly harmful. |
|
|
|
|
|
|
|
|
|
|
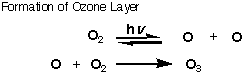 |
|
|
|
|
|
|
|
|
|
|
|
|
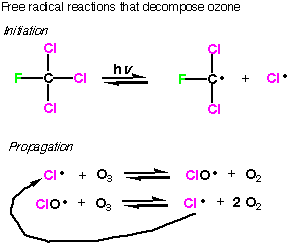
When
CFC’s diffuse into the top of the ozone layer they are exposed to intense
ultraviolet light, which leads to dissociation of carbon-chlorine bonds. This
makes a chlorine radical. (Note that fluorine radicals are not formed because
the C-F bond is so strong.) After initiation, the radical chain is propagated
in two steps, each of which decompose ozone. The chlorine radical produced at
the end of the second step continues the chain, and each cycle destroys 3 ozone
molecules. It is estimated that in atmospheric conditions, each chlorine radical
produced destroys 100,000 molecules of ozone before the chain is terminated.
Thus, it is the radical chain process that makes CFC’s potentially
so dangerous, even in something as vast as the ozone layer. |
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
The true effects are still being studied and the exact amount of damage caused
by CFC’s are still being debated. The ozone hole over the Antarctic
is real and its fluctuations are being analyzed every year. It is important
to take the safe approach, and CFC’s are being phased out all over the
world. They are being replaced by similar molecules that have been chosen
because they do not dissociate into chlorine radicals. In the replacements,
thre are still fluorine and chloring atoms, but the chlorine and fluorine
atoms are attached to different carbon atoms. This dramatically decreases
the stability of the carbon radical (the attached fluorine atoms stabilize
the radical formed from first generation CFC's, which may be counterintuitive
to you at this point, but trust me) so the propagation steps are no longer
favored and the chain reaction does not start. Hydrofluorocarbons (HFA's),
that is refregerants with no chlorine, are also being used as CFC replacements.
Abstract
of general article on CFC's and ozone layer depletion
Click
here for the abstract of an interesting article describing depletion of ozone
and links to skin cancer in the future
Interesting
article on replacement of CFC's in medical inhalors, such as those commonly
used for treating asthma (i.e. albuterol).
Abstract
of a nice article on skin cancer and repair mechanisms induced by UV light
exposure. |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |