Hemiacetals are generally unstable and are only minor components of an equilibrium mixture of an aldehyde or ketone in water, except in one very important type of compound. When a hydroxyl group is part of the same molecule that contains the carbonyl group, and a five- or six-membered ring can form, the compound exists almost entirely in the cyclic hemiacetal form. Recall that five- and six-membered rings have relatively little ring strain.
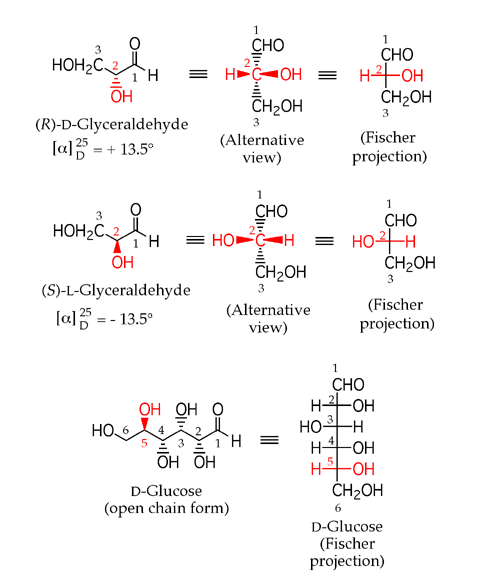
Carbohydrates like glucose exist in solution
as cyclic hemiacetals. Because carbohydrates have several
alcohol groups, they could potentially form rings of different sizes. Generally,
only the most stable (strain free) cyclic structures are produced to an appreciable
extent for a given carbohydrate. The new stereocenter created in a carbohydrate
cyclic hemiacetal structure can have either stereochemical configuration,
and the two stereoisomers are usually in equilibrium with each other. The
carbon atom at the new stereocenter of a carbohydrate cyclic hemiacetal is
given the special name of anomeric carbon
and corresponds to the carbonyl carbon atom in the open chain form. The two
different carbohydrate cyclic hemiacetal stereoisomers are called anomers
and are labeled as alpha or beta, depending on
whether the hemiacetal -OH group is on the same side of the ring as the terminal
-CH2OH substituent (beta anomer) or on the opposite
side (alpha anomer). The two anomers equilibrate in aqueous solution, a process
known as mutarotation. The process is catalyzed by acid, since hemiacetal
formation is catalyzed by acid.
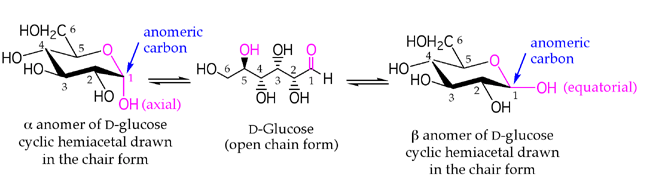
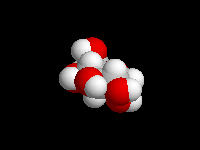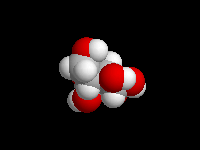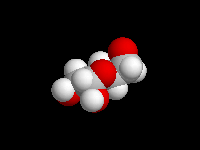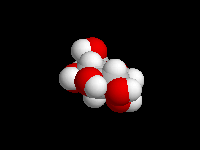
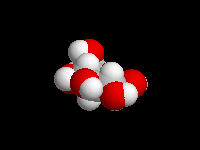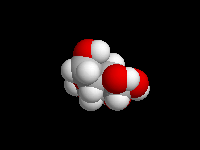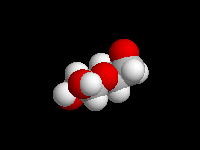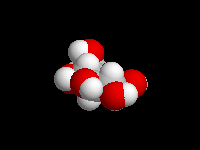
alpha-D-Glucose
(the hemiacetal -OH is axial)
beta-D-Glucose
(the hemiacetal -OH is equatorial)
D-Glucose is the most important carbohydrate in mammalian metabolism. It exists in a six-membered ring cyclic hemiacetal form, as both an alpha and beta anomer. At equilibrium, the beta anomer of D-glucose predominates, because the -OH group of the anomeric carbon is in the more stable equatorial position of the more stable chair structure. In alpha-D-glucose, the -OH group on the anomeric carbon is axial. Remember, for glucose, alpha is axial!
Glucose is important because it represents the mobile source of energy for our cells. Through a variety of enzyme catalyzed reactions (called metobolism), glucose is oxidized, and the energy released from this process is used to provide energy for our cells. A constant glucose concentration in the bloodstream is therefore key to feeling well, and diabetes is a serious condition in which glucose levels are not maintained appropriately due to a breakdown, at one of several possible places, of the insulin system that keeps glucose at proper levels. Excess glucose is stored in a polymer form as glycogen in which the glucose molecules are connected through covalent bonds, and the glycogen is broken down into individual monomeric glucose when needed by the body during excercise or periods of fasting. Note that glycogen is not fat, as fat is a different chemical entity altogether, requiring multiple enzyme reactions to be converted into glucose for our use as an energy source.
Here are some recent articles related to glucose.
The serious health effects of diabetes caused by a lack of control of blood glucose concentration