Click here for a printer friendly pdf version of all of the Golden Rules pages
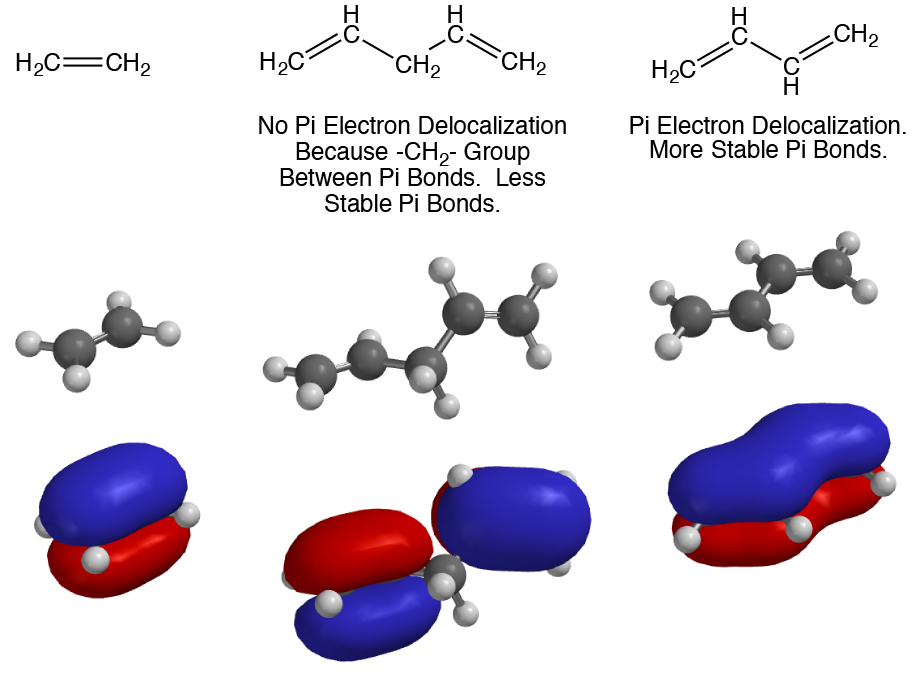
7. Delocalization of pi electron density over a larger area is stabilizing. Pi electron density delocalization occurs through overlapping 2p orbitals, so to take part in pi electron density delocalization atoms must be sp2 or sp hybridized and reside in the same plane. Pi electron delocalization can involve even large numbers of such atoms. Pi electron density cannot delocalize onto or through sp3 hybridized atoms because an sp3 atom has no 2p orbital. Aromaticity is a special type of pi electron density delocalization involving rings and a specific number of pi electrons, and is the most stabilizing form of pi electron density delocalization.