
Click here to download a printer friendly pdf version of this page
Roadmaps
As stated in the syllabus, the “point” of organic chemistry is synthesis, the construction of more complex molecules from simpler ones. By the end of the semester, you will have to solve synthesis problems in which you put together a sequence of several reactions to convert a starting molecule into a product molecule or product molecules. How do you study to prepare yourself for these problems? I believe the best way to review/learn organic chemistry reactions is through the creation of a so-called “roadmap”.
A roadmap is a large piece of paper, the size
of a poster, in which the essential information (i.e. reagents used, stereochemistry,
regiochemistry, key mechanistic steps) about each reaction we study is recorded. To make a
roadmap:
1) Write the names of the key functional groups we study (alkanes, alkyl halides, vicinal dihalides, alkenes, alkynes, alcohols, ketones/aldehydes, carboxylic acids, carboxylic acid derivatives, amines, thiols, ethers, epoxides, various aromatic compounds we will study this semester) spread out over the paper. I have prepared templates for both 320M/328M and 320N as well as a brief chart summary that lists the important reactions from 320M/328M .2) List all reactions as arrows that point from a starting functional group to a product functional group.
3) On the top of the arrow is written the reagents required to carry out the reaction. For example, you would write an arrow from “alkenes” to “alkanes” that has “H2 / Pt, Pd, or Ni” written on top of it to describe catalytic hydrogenation.
4) Just under the arrow write the important features of the mechanism of the reaction, listing the stereochemistry (syn or anti), regiochemistry (Markovinikov, non-Markovnikov), and key mechanistic steps ( i.e. free radical process, E2 elimination, occurs on metal surface, etc.). Note that not all reactions have stereochemistry or regiochemistry considerations to keep track of, but many do and these issues were discussed at the time the reactions were introduced to you. Recall that knowing the exact stereochemistry or regiochemistry of a reaction, if relevant, will tell you exactly which isomer, either which stereoisomer or regioisomer, of a given product is produced (i.e. cis or trans, R or S configuration, primary or secondary alcohol, etc.)
5) Keep track of those few precious reactions that make or break carbon-carbon bonds. I will make a big deal of these in class.
For example, I would have the following written on an arrow from alkenes to
alkanes, describing transition metal catalyzed hydrogenation of alkenes:
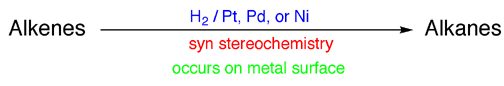
Click here to see
the suggested arrangement of functional groups for a first semester
roadmap (320M/328M), with some representative examples filled in. This
arrangement was chosen to minimize the number of arrows that must
cross each other, although some will. Write the functional groups
in this arrangement on your large piece of paper, leaving plenty of
space between functional groups.
Make sure you use a VERY LARGE sheet of paper, and leave plenty of
room between functional groups.
I recommend you view the map as being roughly analogous to the state of Texas, with the I-35 corridor represented by alkanes (like San Antonio in the south), followed by alkyl halides (San Marcos), alkenes (Austin), vicinal or geminal dihalides (Waco) and alkynes (Dallas). You will find that as you convert different functional groups in synthesis problems, you will often travel along this path. Even more, you will notice that the alkenes, aka Austin, is the most versatile functional group covered in 320M/328M and it is the center of the "action", as is Austin in real life, of course. Most syntheses involve alkenes in one step or another (just like Texans generally find some reason to go through Austin whenever traveling within the state).
By the time you have completed this task for all the reactions we learn,
you should be struck by how alkenes (aka Austin) really is at the center
of the chemistry you learned in 320M/328M. That is, alkenes have the
most arrows pointing toward them and away from them. You should now
be ready to study for synthesis. For example, you should be able to
see that if you were asked to synthesize an alkene from a corresponding
alkane, you cannot do it directly. Rather, you would break it into
two steps (alkane to alkyl halide using X2 and light or heat, followed
by an E2 elimination of the alkyl halide using base such as KOtBu making
sure to follow Zaitsev's rule). I call this a roadmap, because synthesis
comes down to moving from one “location” (i.e. functional group)
on the roadmap, to a different location, and the arrows (i.e. “roads”)
tell you how you must travel. For 320M/328M, "I-35" is the most
often traveled route in synthesis problems!
To assist in this process, I have created a brief chart summarizing the important reactions from 320M/328M. Click here to see the chart. Note that I have not included the important mechanistic points of each reaction on the chart such as regiochemistry, stereochemistry and the key transition state or intermediate. You must recall those on your own and add them to your roadmap.
For this semester, use the 320N roadmap template. Fill in reactions as we go so you continually see the "big picture" of how each individual reaction relates to the others we have learned. Keep your 320M/328M roadmap handy as you will always be responsible for those reactions as we go through this semester. Notice that this semester the carbonyl chemistry is the center of the map, and we make many, many more carbon-carbon bonds!
A final comment. Many students find it helpful to keep the large roadmaps posted on their wall. This will not only reinforce the material several times a day as you glance at it, it will also greatly impress your friends, parents, and roommates who do not know organic chemistry. As you go through the semester in 320N, add new reactions to the roadmap after each lecture. It will help provide a big picture understanding of the chemistry you are learning!
Here are some finished roadmaps from the textbook for your reference.