
Click here for a printer friendly pdf version of this page
The most important question in chemistry is "Where are the electrons?"
The answer is that electrons are generally in higher amounts around the more electronegative atoms (e.g. F, Cl, O, N) of a molecule. The electronegative atoms pull electron density away from the less electronegative atoms (e.g. C, H) to which they are bonded. Thus, understanding electronegativities provides a simple method of deciding which portions of a molecule have a relatively high electron density, and which portions have a relatively low electron density. Molecules with areas of high and low electron density are referred to as ‘polar’ molecules. Molecules that have relatively uniform electron densities are referred to as ‘nonpolar’ molecules.
Electronegativities of the atoms we will normally encounter in organic chemistry:
H = 2.1, C = 2.5, S = 2.5, Br = 2.8, N = 3.0, Cl = 3.0, O = 3.5, F = 4.0
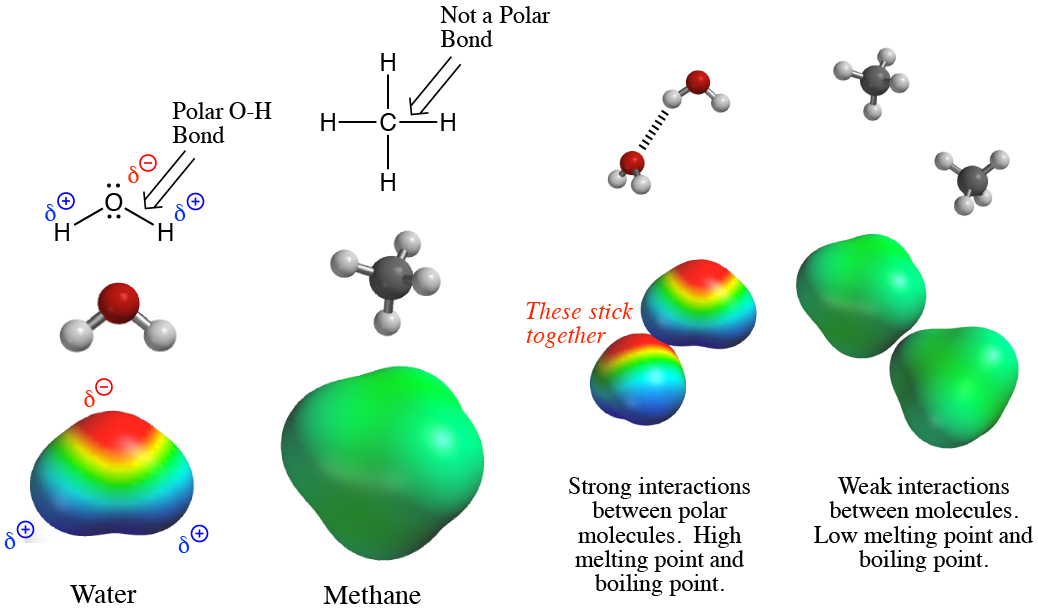
Physical properties such as melting points, boiling points and solubilities can be understood by analyzing distributions of electron density. All things being equal, molecules that are more polar will ‘stick’ to each other better, increasing both the melting points and boiling points (it takes more energy and thus heat to break apart the interactions between ‘sticky’ molecules). This is because unequal distributions of electrons in a polar molecule produce corresponding partial charges (remember that electrons are negatively charged), and opposite partial charges attract each other, making the molecules stick together. Nonpolar molecules do not have these partial charges, so they do not stick to each other as well. Also, polar molecules dissolve in polar solvents, again because of the attractions between partial charges on the solvent and the polar molecules. Nonpolar molecules dissolve in nonpolar solvents.
Relative acidities are also understood on the basis of electron distribution. When a molecule loses a proton, it is acting as an Brønsted-Lowry acid. Because a proton has a positive charge, losing a proton generally leaves a negative charge behind. A molecule will be more willing to give up a proton (i.e. it will be a stronger acid) the better the negative charge can be absorbed after the proton leaves. A molecule can better absorb a negative charge if it has more electronegative atoms that can accommodate the negative charge. Later you will see how important it is to be able to predict relative acidities of molecules in order to predict important attributes such as relative ‘leaving group’ abilities.
Finally, and most importantly, understanding where the electrons are located in a molecule allows for the understanding/prediction of reactions. As you will learn, the vast majority of reactions involve the electron-rich portions of one molecule or species (the so-called ‘nucleophile’) reacting with the extremely electron deficient portion of another molecule (the so-called ‘electrophile’). Being able to understand and predict reactions, then, comes down to being able to predict the locations of these reactive sites (i.e. where are the electrons?).
The bottom line. Understanding where electrons are in molecules (i.e. around the more electronegative atoms) allows you to understand/predict physical properties, acidities and reactions of molecules. It is just that simple. In short, you will understand organic chemistry, it will make sense to you, it will be far easier than trying to memorize a bunch of facts and your organic chemistry classes will be enjoyable. O.K., maybe the word ‘enjoyable’ was a stretch here, but it will be less painful than you are expecting. I guarantee it!