Mechanism Miscues to Avoid: Common Mistakes Students Make When Writing Mechanisms
Throughout this course arrow pushing is used to indicate the flow of electrons in the various organic reaction mechanisms that are discussed. A few simple rules for properly performing arrow pushing were introduced in Section 6.2. In this Appendix we examine some of the most common mistakes that students make when first learning arrow-pushing methods and tell you how to avoid them. The mistakes given below are the ones seen most often by the authors during their cumulative dozens of year of experience in teaching Introductory Organic Chemistry.
- Backwards Arrows
- Not Enough Arrows
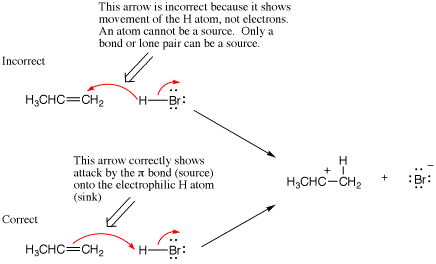
A second common mistake in writing arrow-pushing schemes is to not use enough arrows. This usually results from not keeping track of all lone pairs, bonds made, or bonds broken in a mechanism step. In other words, if you analyze exactly the new position of electrons resulting from each arrow, missing arrows will become evident. In the following example we compare two arrow-pushing scenarios, one of which is missing an arrow. In the incorrect scheme there is no arrow that indicates breaking of the C-H bond of the reactant and formation of the p-bond in the alkene product. Note that when an arrow is missing, the result is commonly too many bonds and/or lone pairs on one atom (see the next section on hypervalency) and not enough bonds or lone pairs on another.
- Hypervalency
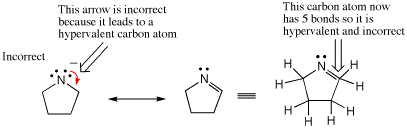
Another frequent mistake when writing arrow-pushing schemes is to expand the valency of an atom to more electrons than an atom can accommodate, a situation referred to as hypervalency. An overarching principle of organic chemistry is that carbon has eight electrons in its valence shell when present in stable organic molecules (the Octet Rule, Section 1.2). Analogously, many of the other most common elements in organic molecules, such as nitrogen, oxygen, and chlorine, also obey the Octet Rule. There are three common ways in which students incorrectly draw hypervalent atoms: 1) Too many bonds to an atom, 2) Forgetting the presence of hydrogens, and 3) Forgetting the presence of lone pairs.
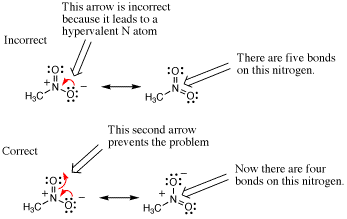
In the following case an arrow is used to depict a potential resonance structure of nitromethane. However, the result is a nitrogen atoms with 10 electrons in its valence shell because there are too many bonds to N. Such mistakes can be avoided by remembering to draw all bonds and lone pairs on an atom so that the total number of electrons in each atoms valence shell is apparent.
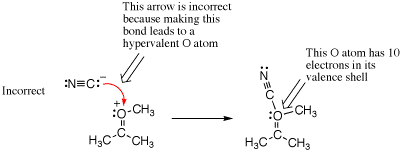
Another common way to make a hypervalency mistake is by forgetting to count all lone pairs of electrons. The following example shows a negatively charged nucleophile incorrectly adding to the formal positive charge on an alkylated ketone. This may look correct because atoms with positive and negative charges are being directly combined, but when counting bonds and lone pairs of electrons, it is found that the oxygen ends up with 10 electrons overall. Hence, this is a mistake.
- Mixed Media Errors
Acids and bases are catalysts, reactants, products, and intermediates in many organic chemistry transformations. When writing mechanisms for reactions involving acids and bases, there are three general rules that will help guide you in depicting the correct mechanism.
- Do not show the creation of a strong acid for a mechanism of a reaction that is performed in strongly basic media.
- Do not show the creation of a strong base for a mechanism of a reaction that is performed in strongly acidic media.
- In strongly acidic media, all the intermediates and products will be either neutral or positively charged, while in strongly basic media, all the products and intermediates will be neutral or negatively charged.
The reason for these rules is that significant extents of strong acids and bases cannot co-exist simultaneously in the same medium because they would rapidly undergo a proton transfer reaction before anything else would happen in the solution.
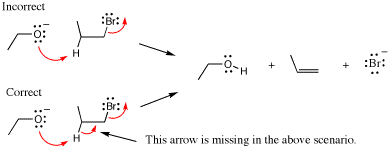
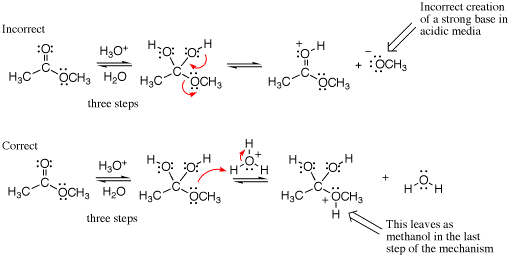
An example of a mixed media error is given below. The first example shows a strong base being created although the reaction is performed under acidic conditions (see conditions over the first equilibrium arrows). Not shown are the three steps that lead to the intermediate drawn. A mistake is made in the arrow pushing because a strong base (methoxide) is generated as the leaving group even though the reaction is run in strong acid. In the correct mechanism, the next step would be protonation of the ether oxygen atom followed by loss of methanol in the last step (not shown) to give a carboxylic acid product.
- Failing to conserve charge
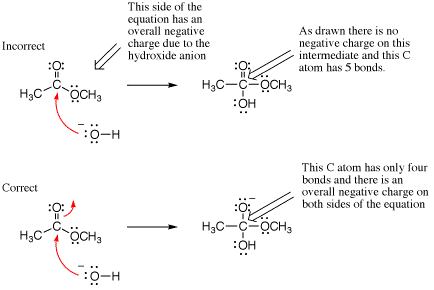
Overall charge must be conserved in all mechanism steps. Failure to conserve overall charge could be caused by some of the preceding errors (hypervalency, failure to draw arrows, mixed media errors), but we mention it by itself because it is always helpful to check that your arrow pushing is consistent by confirming that overall charge conservation is obeyed. In the example shown below, an arrow is missing leading to a neutral intermediate even thought the overall charge on the left side of the equation was minus one. Notice there are five bonds to carbon on the intermediate (hypervalency), providing another obvious indication that something was incorrect in the mechanism step as drawn.