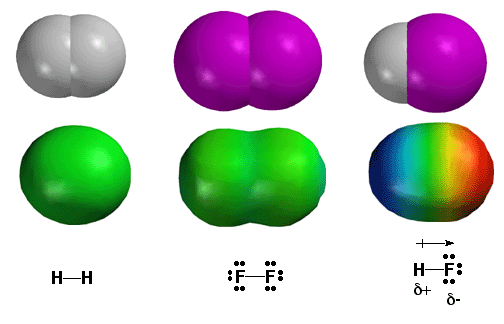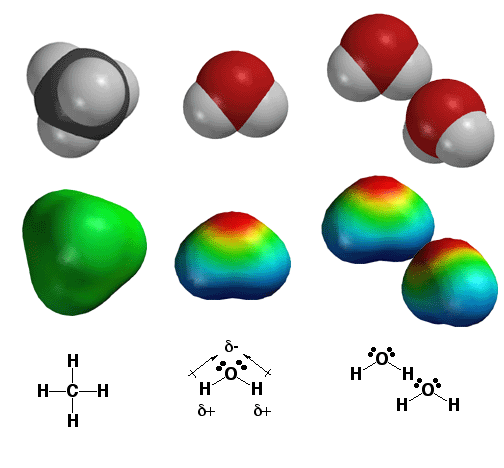Pictures of the Day
1-16-2024
It is important that you come to class
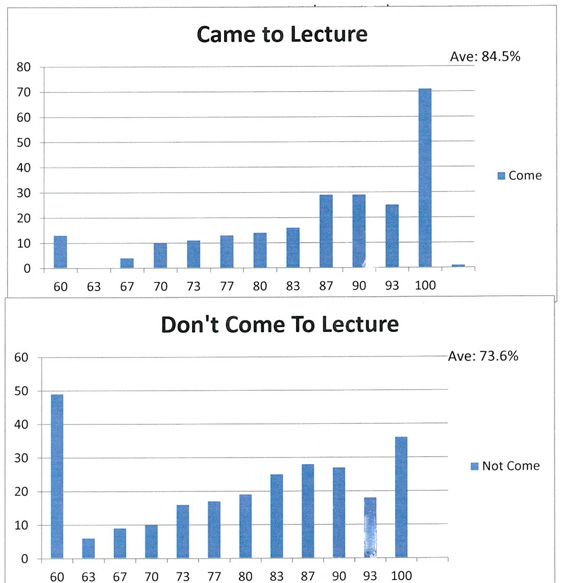
In the spring of 2012, I ran an experiment to gauge the importance of student attendance at lecture. Because my lectures are recorded and available on-line, many students chose to quit coming to lecture. I gave an unannounced quiz in the middle of the semester, and we used the responses to take attendance in the class. We then tracked the performance of the students present in lecture that day for the entire semester, as well as those students who chose to remain home that day. As plotted above, students attending class on the day attendance was taken averaged around 85 (a solid B) for their final course grade, while those choosing to not come to that lecture averaged around 74 (a solid C). Perhaps more important, the majority of students destined to receive an A for the course were present in lecture, while the overwhelming majority of students destined to fail the course, get an F grade, were not. Bottom line: COME TO CLASS IF YOU WANT TO DO WELL!


Has learned enough to UNDERSTAND the fundamentals (Golden Rules of Chemistry, Where are the Electrons, etc.) so now has intuitive feel for Organic Chemistry. Does not have to work as hard and can stay on top of the class. He looks at a reaction and just sees the answer because he now has organic chemistry intuition.
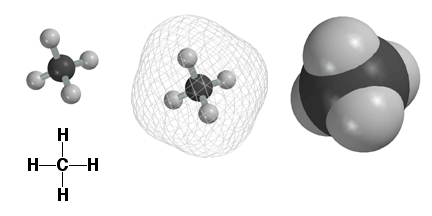
Atoms are surrounded by electrons and we use the computer to plot molecules to reflect where the electrons are. Above are three different computer images of the methane molecule CH4. The image on the upper left shows the C and H atoms as small balls connected by sticks, i.e. the bonds. This is a nice picture, but does not really show where the electrons are, since they are found farther away from the atomic nuclei. Shown in the middle above is a mesh surface under which the electrons can be found. On the upper right is shown a solid surface, color coded by atom type. This type of representation is referred to as a space filling model. The space filling models are a better way to think about molecules, since they give a realistic picture of molecular size, electrons and all. Refer to the atom type chart to remind yourself which atoms have which colors.
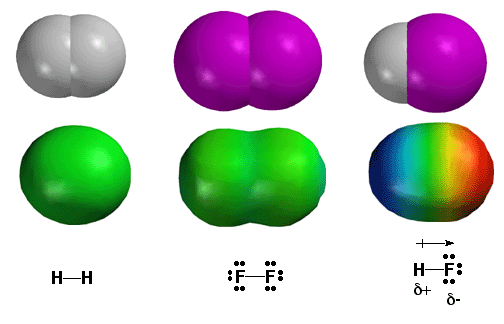
The most important concept in chemistry is that some atoms are more electronegative than others, in other words, they “hog” more of the electrons in a covalent bond. Thus, electronegativity answers the most important question in chemistry. Electron density distribution in a molecule can be quantitatively displayed in color coding using so-called electrostatic potential surfaces calculated by computer. Shown above are three simple diatomic molecules. On the left is H2, with a single bond between the H atoms. Since two H atoms have the same electronegativity, they share the electrons evenly. This is depicted as a green color on the electrostatic potential surface plot. In the middle is the F2 molecule. Again, since both the F atoms of the bond have the same electronegativity, the electron density is shared evenly between the two atoms and the electrostatic potential plot is green. On the right is the H-F molecule. Fluorine is much more electronegative than hydrogen, so the majority of electron density resides on the fluorine atom. High electron density around the F atom is depicted as the red color on the electrostatic potential surface above. The hydrogen atom has less electron density, depicted as blue color to indicate the absence of electron density. This type of covalent bond, with the electrons shared unevenly is called a polar covalent bond (difference in electronegativities of bonded atoms is greater than 0.5). The polarity of the bond is quantified by using the vector quantity of dipole moment, represented by the arrow symbol above the bond. Note that since electron density has negative charge, the fluorine atom has a partial negative charge (d-) leaving a partial positive charge (d+) on hydrogen.
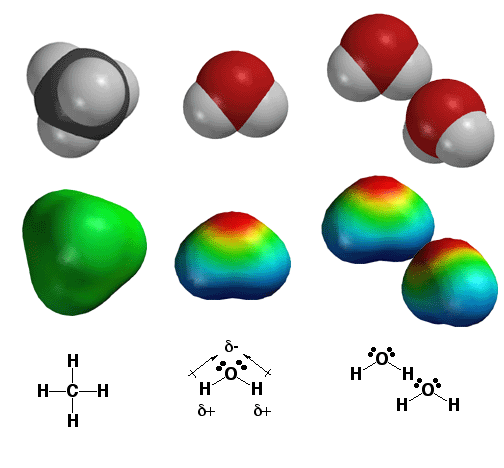
The reason “where are the electrons” is the most important question in chemistry is that knowing the answer allows you to PREDICT important chemical properties and reactions by analyzing molecules for areas of high and low relative electron density. On the left is the methane molecule, with only non-polar covalent bonds as indicated by the green color on the electrostatic potential surface. In the middle is the water molecule, which has two very polar covalent bonds as indicated on the label with bond dipole moment symbols. The electrostatic potential surface shows how the majority of electron density (red color) resides on the oxygen atom, with a lack of electron density (blue color) on the hydrogen atoms. Since electron density has negative charge, this means that the oxygen atom has partial negative charge (d-), and the hydrogen atoms have partial positive charge (d+). Since opposite charges attract, this means that in water the oxygen atoms of one molecule attract the hydrogen atoms of another to form a so-called hydrogen bond (more details on that later) as shown on the right. The bottom line is that all of this attraction literally “sticks” the water molecules together, so that water does not boil until relatively high temperature (100°C). Since the methane molecules do not have polar bonds and thus no partial charges, the molecules are not attracted to each other in the same way, the molecules do not stick together nearly as well and methane is a gas at room temperature (it boils at very low temperature)!. The bottom line is that knowing where the electron density is in molecules, you can predict and/or understand properties such as relative boiling points!