Pictures of the Day
4-2-2024
The Molecular Orbital Theory Moment-Review
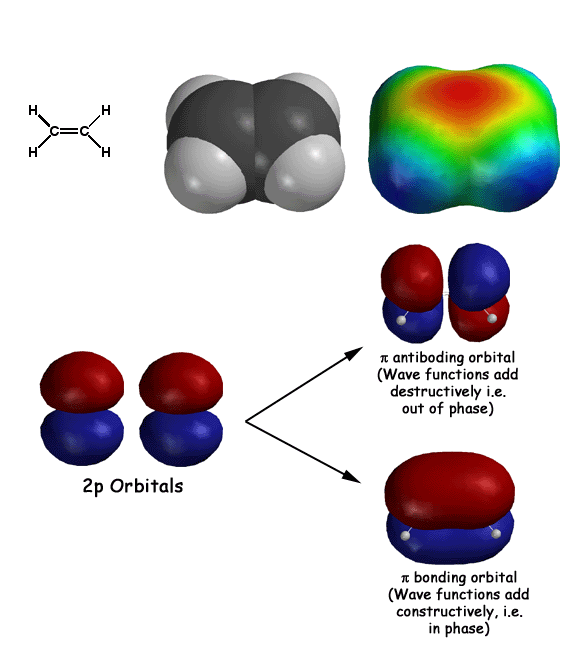
Some basics of molecular orbital theory as it relates to pi systems. First electrons behave as waves, so they are described by wave functions (also known as orbitals). Adjacent atomic 2p orbitals add according to wave mechanics, namely constructively (in phase) and destructively (out of phase). The in phase addition creates the lower energy bonding molecular orbital (the "hotdog bun"), while the out of phase addition results in the higher energy antiboding orbital. The two electrons end up in the bonding orbital, resulting in what we refer to as a pi bond. Note how these pi bonding electrons in the pi bonding orbital are held out and away from the nuclei, so they provide some partial negative charge (red color) to the electrostatic potential surface. Since electrophiles can interact with these electrons, alkenes react as weak nucleophiles.
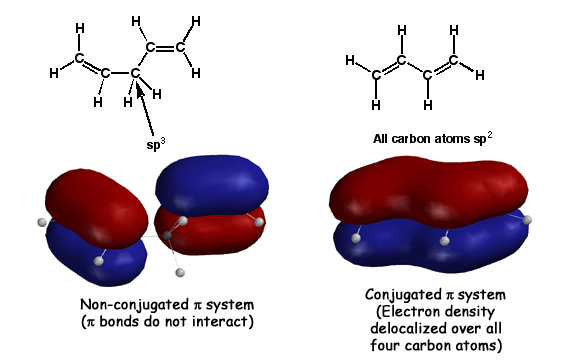
Conjugation occurs when adjacent pi bonds overlap, creatiing a large "pi way" for the electrons to delocalize into. The molecule on the right, butadiene, has adjacent pi bonds that overlap to form the large molecular orbital shown. Such overlap of pi bonds is referred to as "conjugation". This is stabilizing compared to isolated pi bonds because pi electrons prefer to be delocalized over as large an area as possible(Golden Rule #7). Since these delocalized pi electrons are in a more stable situation, they are less reactive with electrophiles. The molecule on the left, 1,3 pentadiene, has an sp3 atom between the pi bonds. The pi bonds cannot overlap in this case, so there is no delocalization, no stabilization, and the pi electrons are as reactive as any old alkene.