Basicity of Pyrindine v.s Pyrrole. Where is the lone pair?
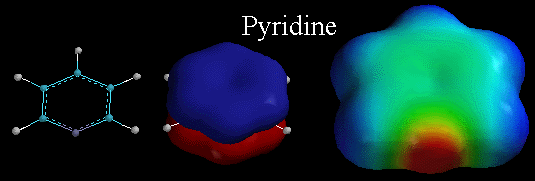
Above, are three diagrams illustrating the characteristics of pyridine. Pyridine is a flat, monocyclic compound with 6 pi electrons, making the molecule AROMATIC. The picture in the middle is the lowest energy pi molecular orbital, emphasizing that the pi electrons in this orbital are distributed over the entire ring. Recall that for pyridine, the lone pair of electrons on the N atom are in an sp2 orbital and are NOT part of the delocalized pi system. Therefore they ARE localized and are available for binding to protons. Thus, the pyridine is BASIC. You can see this by looking at the electrostatic potential surface of pyridine on the right. Notice the localized area of electron density (the deep red color) associated with the lone pair of electrons on the N atom.
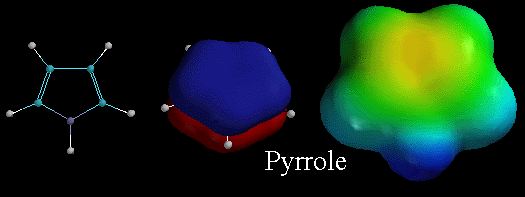
Like pyridine, pyrrole is also an aromatic heterocyclic compound. The middle picture shows the lowest energy pi molecular orbitals, emphasizing that the pi electrons in this orbital are distributed over the entire ring. Recall that for pyrrole, the lone pair of electrons on the N atom are in a 2p orbital and are part of this delocalized pi system. Therefore, these lone pair electrons are delocalized and are NOT available for binding to protons. Thus pyrrole has NO area of localized electron density on nitrogen (no deep red color as shown in the electrostatic potential surface on the right) and so pyrrole is NOT VERY BASIC. Contrast this to the electrostatic potential surface diagram for pyridine which has a localized area of electron density.
Pictures of the Day
4-9-2024
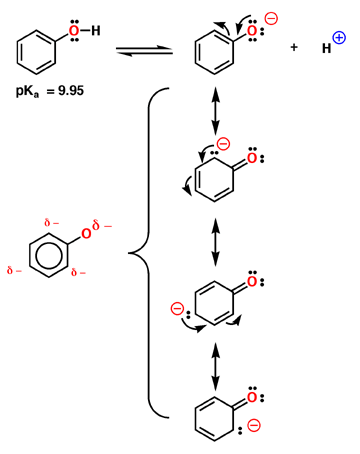
The acidity of phenol - charge distribution in an aromatic ring
Phenol is relatively acidic, with a pKa near 9.95. Compare this to a normal alcohol in which the pKa is closer to 16. Phenol is more acidic because the negative charge of the anion is delocalized around the aromatic ring as described by the 4 contributing structures above. Note that most of the charge is still on oxygen, but enough is delocalized in the aromatic pi system to make a substantial difference. The interesting prediction of these resonance contributing structures is that the negative charge is not distributed evenly around the ring, but remains primarily in the ortho and para positions. This is shown in the electrostatic potential surface in which the negative charge (red color) is primarily ortho and para to the oxygen atom.