Pictures of the Day
4-16-2024
Arenium ion
The Good, the Bad, and the Ugly
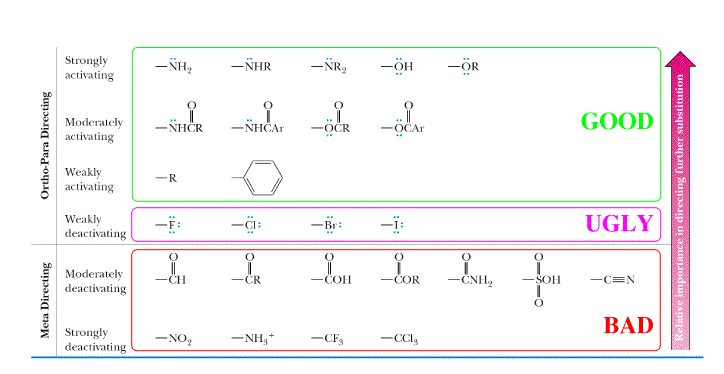
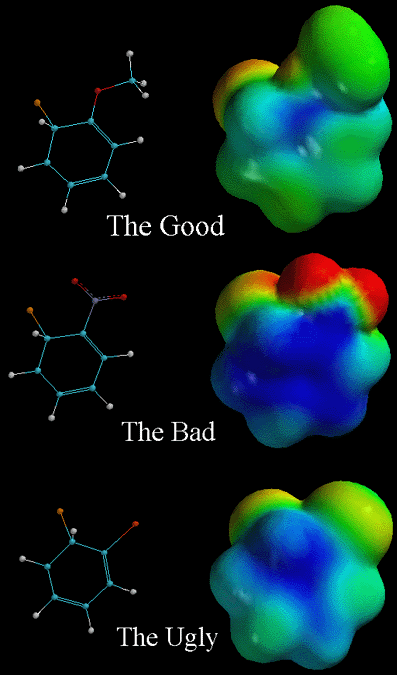
In the pictures above, the arenium ion intermediates of an electrophilic aromatic substitution are shown. In each case, a different ortho substituent is placed on the benzene ring, thereby changing the stability of the intermediate. Remember that placing the electrophile para to the substituent has essentially the same effects as placing it ortho to the substituent. In the pictures, the attached electrophile (Cl+) is orange and the substituent is next to it. The ortho substituent on "The Good" is O-CH3, the substituent on "The Bad" is NO2, and the substituent on "The Ugly" is Cl. As the chart says above, "The Good" groups, which are electron releasing groups, direct WSE's (wicked strong electrophiles) to the ortho and para positions, and are activating. Activating means that this compound will react faster compared to benzene with only H's. This is because the substituent contributes electron density to the intermediate, which during this stage, lacks electron density (is positively charged). This makes the intermediate more stable relative to the arenium ion derived from benzene and it takes less energy to get to this intermediate. Notice how this intermediate (the intermediate directly below the arenium ion derived from bezene) is less blue (less positive charge) than the arenium ion derived from benzene when viewing their respective electrostatic potential surface diagrams. The positive charge of the intermediate is delocalized over more atoms (resonance effects) and the substituent contributes electron density to the intermediate. The next picture illustrates why "The Bad" is less stable than "The Good," "The Ugly," and the unsubstituted arenium ion derived from benzene. Notice that there is more positive charge (blue color) and it is very localized. This is because the NO2 group is very electron withdrawing. When the electrophile is placed in the meta position (not shown), the intermediate is slightly more stable than the intermediate shown. This is why "The Bad" group is meta directing, but it is still strongly deactivating. The substituents in "The Ugly" group are deactivating but not as deactivating as "The Bad" group. They are deactivating in that they react slower than benzene. The "Ugly group" is deactivating because halogens withdraw electron density from the benzene ring (inductive effect), destabalizing the intermediate. However, these substituents are ortho/para directing because the halogens can donate a lone pair of electrons, leading to resonance stabilization, but only at the ortho/para positions (resonance effect). The bottom line is that analyzing the stablilities of the various possible intermediates in an electrophilic aromatic substitution allows you to understand whether a given substituent is activating or deactivating and indicates where the electrophile reacts (ortho/para vs. meta). It all comes down to stabilizing or destabilizing the positive charge!


