Increasing acidity with the inductive effect
Pictures of the Day
2-6-2025
The Enolate Anion: Stabilized by Resonance
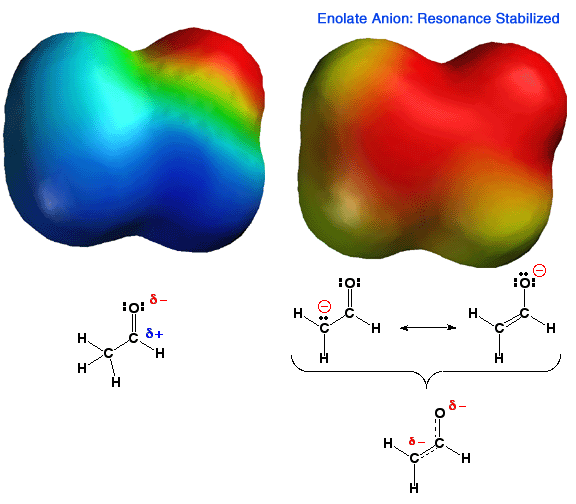
Shown above is the electrostatic potential energy surface for an enolate ion. Note how the negative charge, (red color) is distributed over both the alpha carbon atom and the oxygen atom of the enolate. You should be able to draw resonance forms that describe this charge distribution. This delocalization of charge makes this anion more stable than an anion with a localized charge. Thus, carbonyl groups that have an alpha proton are MUCH more acidic than other types of C-H bonds such as alkanes or alkenes. In addition, enolates are very reactive nucleophiles, being able to react at either the alpha carbon atom or the oxygen atom. That is, they are not only highly reactive, they are also "schizophrenic" nucleophiles. Because carbon-oxygen double bonds are stronger than carbon-carbon double bonds, as well as other factors like solvation etc., reactions with electrophiles, especially carbon electrophiles, occur primarily at the carbon atom of the enolate.
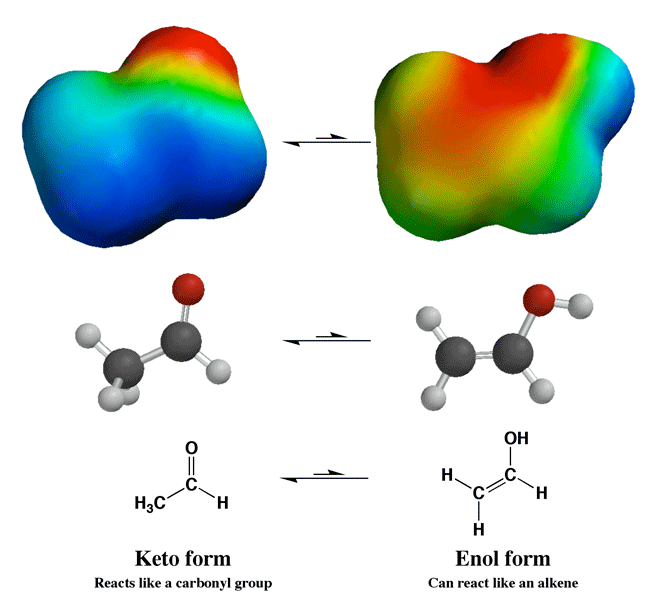
Keto-Enol Equilibrium Changes the Personality of the Carbonyl Compound
Even though keto-enol equilibrium strongly favors the keto form, the enol form is important in some reactions like alpha halogenation. The key idea here is that the enol reacts like an alkene with reagents like Br2 or Cl2 to give alpha halogenation. Acid catalyzes the keto-enol equilibrium, so adding acid to these reactions increases the rate of enol formation, and therefore the rate of the reaction.
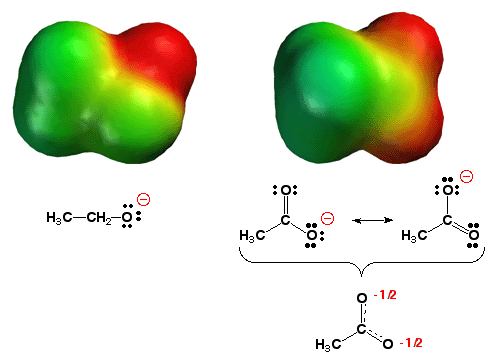
Carboxylate anion - stabilized by resonance delocalization
When comparing acid strengths, it is usually helpful to compare the structures of the negatively-charged conjugate bases produced when the acids are deprotonated. When comparing similar acids, the more stable the conjugate base, the more acidic the parent acid. Mother Nature hates localized charges, so the more delocalized a charge, the more stable the ion. Thus, a more stable anionic conjugate base can usually be identified as the one that has the negative charge distributed over more atoms. This is a huge effect. The carboxylate anion (above right) has the negative charge (red color) distributed over both oxygen atoms, while the alkoxide anion (above left) has the negative charge (darker red color) localized on only one oxygen atom. Thus, it is no surprise that acetic acid is 12 orders of magnitude (roughly the size of the national debt in dollars!) more acidic than ethanol.
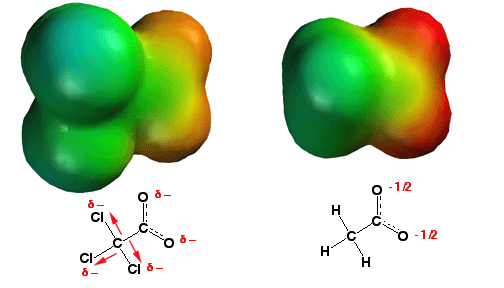
Any group of atoms that can help delocalize the negative charge of a conjugate base makes the parent acid more acidic. The chlorine atoms on trichloroacetic acid (above left), being electronegative, withdraw some electron density away from the oxygen atoms of the carboxylate (a so-called "inductive effect"), thus helping to delocalize the negative charge. This can be seen in the electrostatic potential surfaces in that the oxygen atoms of the trichloroacetic acid carboxylate have less negative charge (red color) than those of the acetic acid carboxylate (on the right). This charge delocalization stabilizes the anion, explaining why trichloroacetic acid is about 4 orders of magnitude more acidic than acetic acid!