This movie depicts an E2 elimination reaction between methoxide (H3CO -) and 2-chlorobutane to form methanol,2-butene, and a chloride anion . In the E2 reaction, a new bond is formed between the base, H3CO-, and a proton from the 2-chlorobutane, while simultaneously the carbon-chlorine bond is broken and a new pi bond is being formed on the butane species. The departing chloride anion is referred to as the leaving group.
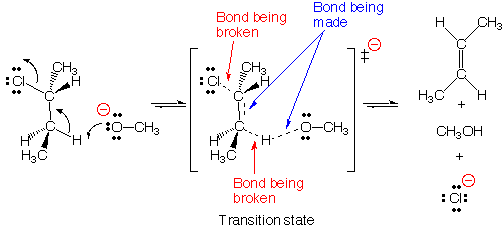
The term E2 stands for Elimination reaction, 2nd order (also
called bimolecular). According to the E2 mechanism, there is a single transition
state because bond-breaking and bond-making occur simultaneously. Reactions
such as the E2 reaction in which atoms are lost from two adjacent atoms of
molecule are classified under the broader category of beta-elimination
reactions.
Bimolecular reaction A bimolecular reaction, such as the
E2 reaction is one in which two reactants take part in the transition state
of the slow or rate-determining step of a reaction. For this reason, the concentrations
of both the base and the alkyl halide are proportional to the observed E2
reaction rate.
Influence of alkyl halide structure on reactivity Secondary alkyl
halides such as 2-chlorobutane can react by either the E2 or SN2
reaction pathways to varying degrees depending on the nature of the nucleophile/base
being used. If a strong base such as methoxide is used, the proton transfer
step is favored and the E2 mechanism redominates as shown in the movie. If
a weak base/strong nucleophile is used such as another halide or the azide
(N3-) anion is used, then substitution according to
the SN2 reaction pathway is favored.
Anti-periplanar geometry For bond-breaking and bond-making
to occur with the proper orbital overlap in the E2 reaction, the proton being
transferred must be located in an anti-periplanar geometry (on opposite side)
with respect to the chloride leaving group. This can be seen in the reaction
movie by examining the direction of the approaching base molecule, and trajectory
of departing chloride anion.