This movie depicts an SN2 reaction between the hydroxide
anion (HO-) and methyl chloride . In this reaction, a new bond is formed between
the nucleophile, HO-, and the carbon atom, while the carbon-chlorine
bond is broken. The departing chloride anion is referred to as the
leaving group. The species being attacked by the nucleophile, namely
methyl chloride, is referred to as the electrophile.
The term SN2stands for Substitution reaction,
Nucleophilic, 2nd order (also called bimolecular). According to the
SN2 mechanism, there is a single transition state because
bond-breaking and bond-making occur simultaneously. Notice that for this to
occur, the nucleophile must approach from the backside of the carbon-leaving
group bond (so-called backside attack ). Look for the backside attack in the
movie.
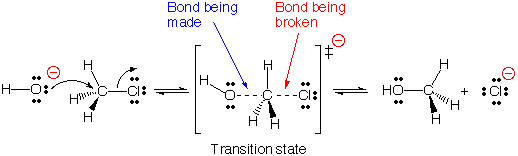
Notice that there is not intermediate in an SN2 reaction, just a transition state. A transition state has no real lifetime, it is the highest energy point on the reaction coordinate as starting materials transition into products.
Bimolecular reaction A bimolecular reaction, such as the
SN2 reaction, is one in which two reactants take part
in the transition state of the slow or rate-determining step of a reaction.
For this reason, the concentrations of both the nucleophile and the alkyl
halide are proportional to the observed SN2 reaction
rate.
Nucleophilicity Because the nucleophile is involved in the
rate-determining step of SN2 reactions, stronger nucleophiles
react faster. Stronger nucleophiles are said to have increased nucleophilicity.
In the gas phase, there is a correlation between increased relative nucleophilicity
and increased base strength, although there are many exceptions to this trend
in solution (see below). In general, within a period of the periodic table,
nucleophilicity increases from right to left. Furthermore, for different reagents
with the same nucleophilic atom, an anion is a better nucleophile than a neutral
species.
Solvent effects In solution the comparison of nucleophilicity
vs. basicity is complicated by solvation effects. Polar aprotic solvents (e.g.
acetonitrile, dimethylsulfoxide, dimethylformamide, etc.) are good at solvating
cations, but not anions. As a result, the nucleophiles are not highly solvated,
and the relative order of nucleophilicities is similar to that observed in
the gas phase. Polar protic solvents (e.g. alcohols, water) are much better
at solvating anions, so nucleophiles are highly solvated in these solvents.
The relative order of nucleophilicities can be changed dramatically by this
solvation. Solvation of nucleophiles by polar protic solvents also inhibits
the nucleophile’s ability to take part in an SN2
reaction, so SN2 reactions are much slower in polar
protic solvents compared with polar aprotic solvents.
Inversion of stereochemistry If the halide leaving group
is attached to a stereocenter, the configuration of the stereocenter is inverted
during an SN2 reaction. This is because the nucleophile
enters from the opposite side of the molecule as the departing group (“backside
attack”), thus the molecule inverts analogous to an umbrella inverting
in the wind. You can see this molecular inversion in the above movie, but
in this case, the inversion does not influence stereochemistry since neither
the reactants nor products are chiral.
Inhibition by steric hindrance SN2
reactions are particularly sensitive to steric factors, since they are greatly
retarded by steric hindrance (crowding) at the site of reaction. In general,
the order of reactivity of alkyl halides in SN2 reactions
is: methyl > 1° > 2°. The 3° alkyl halides are so crowded
that they do not generally react by an SN2 mechanism.