|
|
|
|
|
When
there is restricted bond rotation, as in alkenes or cyclic structures, H atoms
bonded to the same C atom may not be equivalent, especially if the molecule
is not symmetrical. Non-equivalent 1H nuclei on the same C atom will couple
to each other and cause splitting. This is referred to as geminal coupling.
Geminal coupling constants are generally small, on the order of 0-5 Hz. This
is most common in unsymmetrical alkenes and cyclic molecules. Because of the
restricted rotation about C=C bonds, the alkenyl (vinylic) H atoms of unsymmetrical
alkenes are not equivalent (i.e. they are in unique chemical environments).
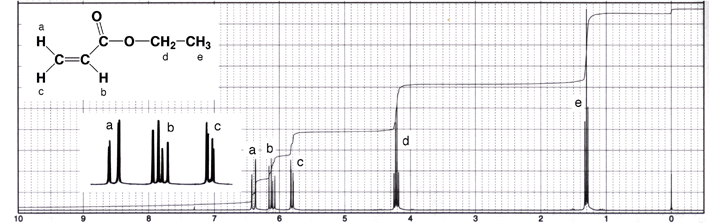
For example, ethyl propenoate (ethyl acrylate) is an unsymmetrical terminal
alkene, and therefore the three alkenyl H atoms are non-equivalent. As a result,
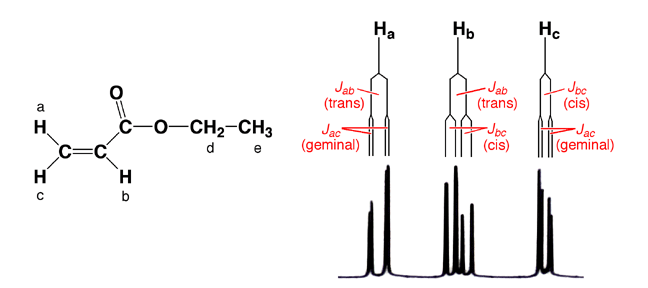
they all couple with each other. In alkenes, trans coupling generally results
in larger coupling constants (Jtrans = 11-18 Hz) compared to cis coupling (Jcis
= 5-10 Hz), with geminal coupling being by far the smallest (Jgem = 0-5 Hz).
Unless a high resolution spectrum is taken, the geminal coupling constant is
so small that it is often difficult to see in terminal alkenes. In the spectrum
of ethyl propenoate below, the geminal coupling is only seen upon close inspection
of the signals labeled “a” and “c”. You should be able
to recognize the characteristic ethyl group pattern of a quartet integrating
to two H atoms (-CH2-, Hd) and a triplet integrating
to three H atoms (-CH3, He). Tree diagrams are provided
underneath the spectrum to help decipher patterns of the alkenyl signals. |
|
|
|
|
|
|
|
Cyclic
structures often exhibit restricted rotation about their C-C sigma bonds, and
can have constrained conformations. The result is that the two H atoms on –CH2-
groups in cyclic molecules can be non-equivalent, leading to complex spin-spin
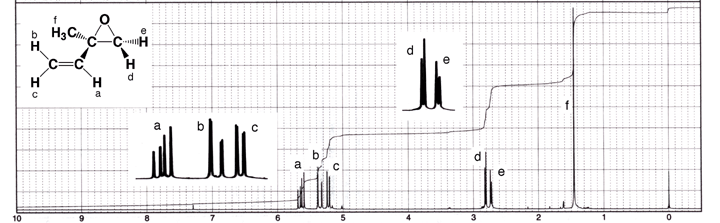
coupling. Substituted epoxides such as 2-methyl-2-vinyloxirane provide a good
example as shown below. The two H atoms on the three-membered epoxide ring
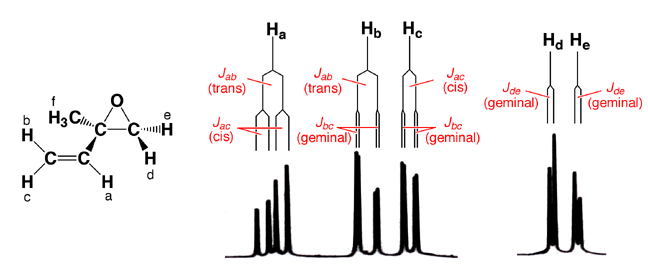
are non-equivalent. Hd is cis to the vinyl group and trans to the methyl group,
while He is the reverse. Since they are in different chemical environments,
they are non-equivalent and exhibit geminal coupling. The geminal coupling
constant is small but discernable in the spectrum as the signals for both Hd
and He are doublets. Vinyl H atom Ha is split by both Hb (trans coupling) and
Hc (cis coupling), giving rise to a doublet of doublets, or four peaks. Hb
is split by Ha (trans coupling) along with Hc, the latter geminal coupling
constant is so small that it is barely discernable at this resolution. Hc is
split by Ha (cis coupling) as well as Hb (geminal coupling). The singlet near
1.5 ppm that integrates to three H atoms is the methyl group labeled as “f”. |
|
|