|
|
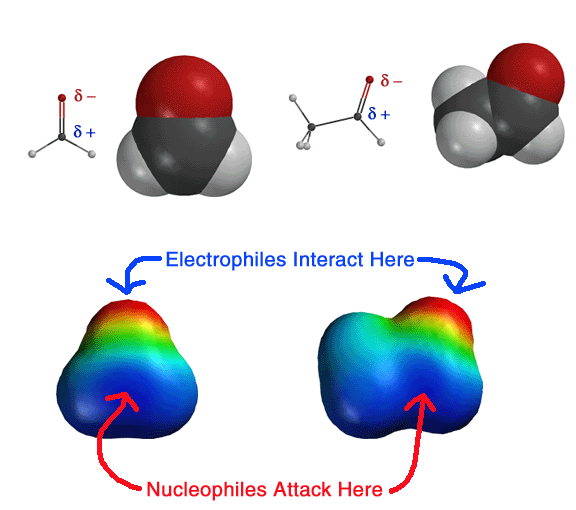
Shown
above is the electrostatic potential surface of a carbonyl, in this case
formaldehyde (left) and acetaldehyde (right). This picture is most of what
you need to remember about carbonyls in terms of nucleophilic attack. The
electrostatic force will direct nucleophiles to the carbonyl carbon atom,
while electrophiles (Lewis acids and protons) will be directed to the oxygen
atom. The pi bond will break upon nucleophilic attack to create the ubiquitous,
always present, never to be forgotten, can't live without it, gotta have
it, gotta love it, TETRAHEDRAL INTERMEDIATE. The main differences between
the carbonyl mechanisms involve the timing of protonation and/or whether
there is a leaving group (Cl-, -OCH3) attached to the
carbonyl. These latter statements will make a lot of sense to you very soon! |
|
|
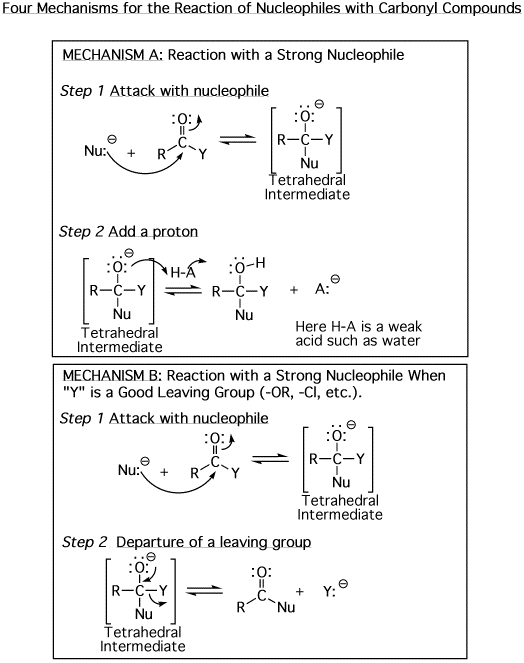
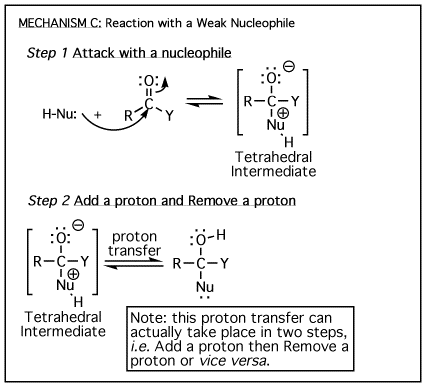
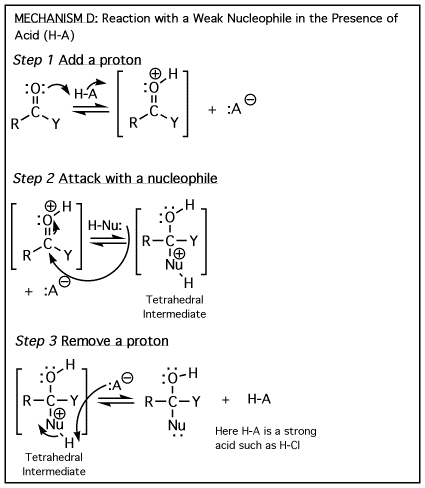
The
above four mechanisms describe all of the different reactions you will see
for carbonyl species such as aldehydes, ketones and carboxylic acid derivatives.
They are really just various combinations of the four mechanism elements A)
Add a proton, B) Remove a proton, C) Attack with a nucleophile, and D) Departure
of a leaving group. They all have a tetrahedral
intermediate, and they all involve a nucleophile attacking the carbon atom
of a carbonyl. The difference comes down to whether there is a leaving group
involved, then the timing of protonation/deprotonation. |
|
|
|
|