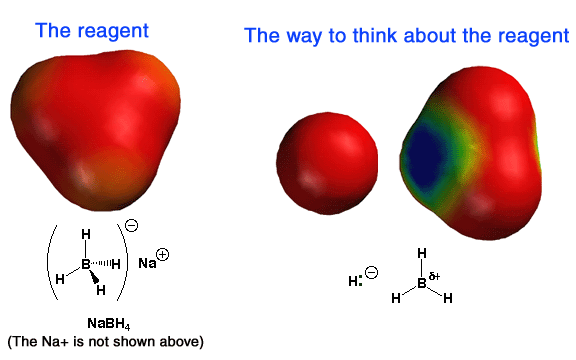
|
Above
are electrostatic potential surfaces that explain a great deal about the reactivity
of NaBH4 (and by analogy LiAlH4, with the Li cation replacing
the Na cation and Al replacing the B atom).
The best way to think about NaBH4 is
as an H- atom (a so-called hydride ion) bonded to the Lewis acid BH3,
as indicated in the middle panel above on the right. This way of thinking helps
explain why the NaBH4 reagent reacts by donating a nucleophilic
H- atom to electrophiles such as the carbon atom of carbonyls. On the left
is shown the actual structure of BH4-; notice the oveall
negative charge (red color). On the right for comparison is BH3,
showing a partial positive charge (blue color) on the boron atom and a partial
negative charge (red color) on the hydrogen atoms, consistent with the electronegativies
of those types of atoms. The partial positive charge on boron explains its
Lewis acid (electron pair seeking) properties, i.e. why it forms such a nice
complex with the negatively-charged oxygen atom of the tetrahedral intermediate. |