|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
 |
|
 |
|
 |
|
|
|
|
|
|
|
|
 |
|
 |
|
|
|
|
|
|
|
|
|
|
|
Pictures
of the Day 2-18-08
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
Enolate Anion: Stabilized by Resonance |
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
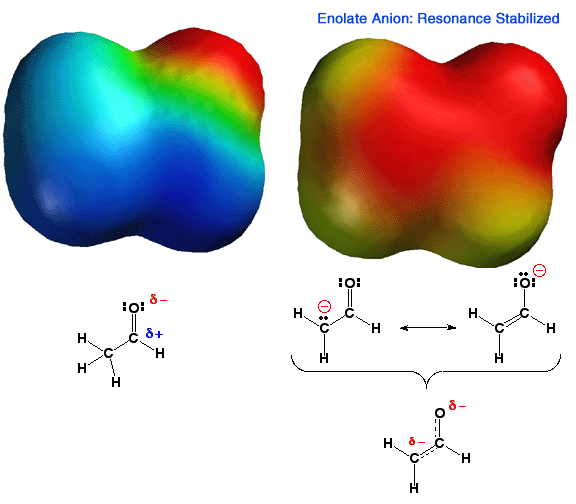
Shown
above is the electrostatic potential energy surface for an enolate ion. Note
how the negative charge, (red color) is distributed over both the alpha carbon
atom and the oxygen atom of the enolate. You should be able to draw resonance
forms that describe this charge distribution. This delocalization of charge
makes this anion more stable than an anion with a localized charge. Thus,
carbonyl groups that have an alpha proton are MUCH more acidic than other
types of C-H bonds such as alkanes or alkenes. In addition, enolates are
very reactive nucleophiles, being able to react at either the alpha carbon
atom or the oxygen atom. That is, they are not only highly reactive, they
are also "schizophrenic" nucleophiles. Because carbon-oxygen double bonds
are stronger than carbon-carbon double bonds, as well as other factors like
solvation etc., reactions with electrophiles, especially carbon electrophiles,
occur primarily at the carbon atom of the enolate. |
|
|
|
|
|
|
Keto-Enol
Equilibrium Changes the Personality of the Carbonyl Compound |
|
|
|
|
 |
|
|
|
|
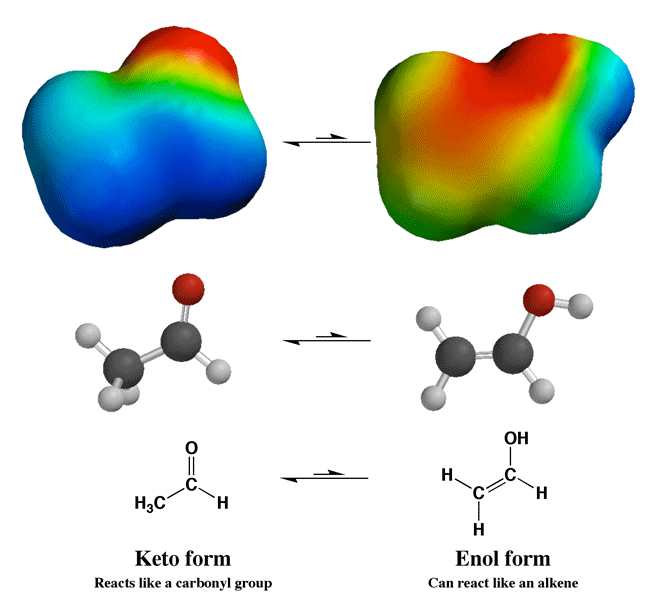
Even
though keto-enol equilibrium strongly favors the keto form, the enol form is
important in some reactions like alpha halogenation. The key idea here is that
the enol reacts like an alkene with reagents like Br2 or
Cl2 to give alpha halogenation. Acid catalyzes
the keto-enol equilibrium, so adding acid to these reactions increases the
rate of enol formation, and therefore the rate of the reaction. |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |


