|
|
|
|
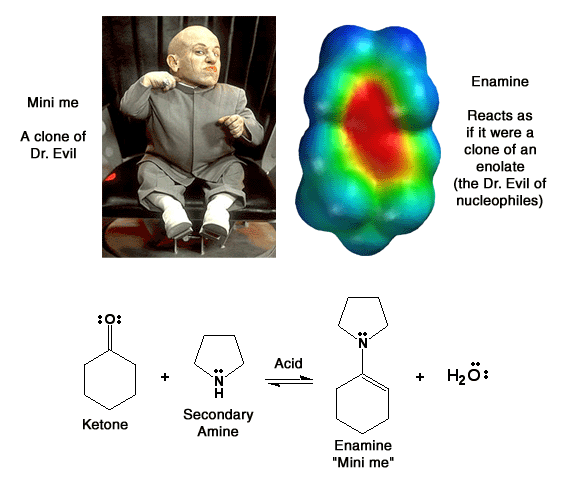
Enamines
("mini me") are molecules derived from ketones and secondary amines,
generally pyrrolidine. They react
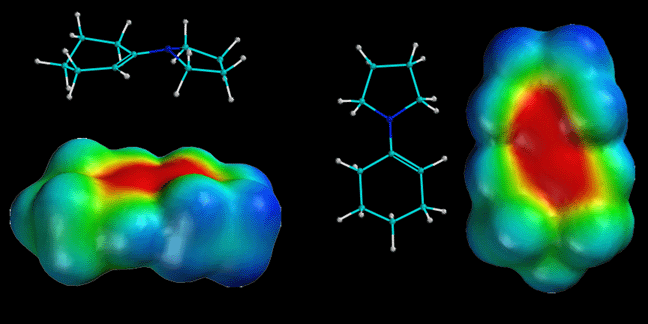
as nucleophiles analogous to enolates ("Dr. Evil"). Note
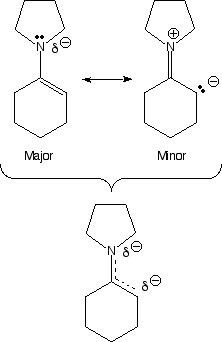
how there is considerable partial negatve charge (red color) on the beta-carbon
atom. This is because of the minor resonance contributor that places negative
charge at that position. This partial negative charge makes this carbon nucleophilic,
exactly analogous to an enolate. Note that the nitrogen atom is also red,
even though the minor contributing structure has a positive charge on the
nitrogen. This is not a contradiction because the major resonance contributor
has partial negative charge on the nitrogen (due to the lone pair of electrons
on nitrogen), and this partial negative charge more than compensates for
the small amount of positive charge indicated in the minor contributor. The
nitrogen atom does not react as a nucleophile because that would create a
less stable product (no motive) compared to reaction at carbon. Complex
arguments aside, the important things to remember about
enamines are 1) they are formed under mild conditions (it is just a simple
Schiff base formation), 2) they react as nucleophiles analgous to enolates
woth both alkyl halides and acid chlorides and 3) they are reversibly formed
and can be taken off by H3O+ after the reaction. Yea
baby!!! |