|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
 |
|
 |
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pictures
of the Day 4-09-08
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Benzene
and Aromaticity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
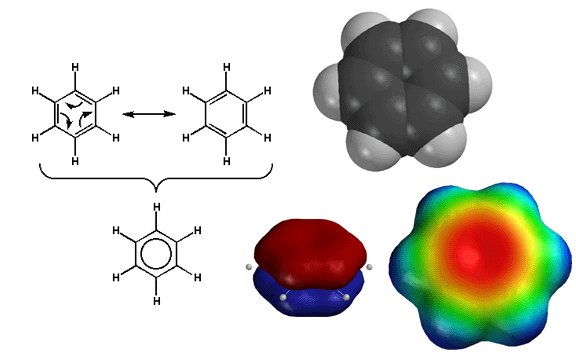
Benzene
is the special case in which all the 2p orbitals are contained within a ring. The
resulting bagel-shaped pi orbital is delocalized over the entire ring. When
certain criteria are met (the Huckel conditions) this is extraordinarily
stabilizing. This type of stabilization is referred to as "aromaticity",
and benzene is said to be aromatic. As a result, the pi electrons of benzene
are relatively unreactive compared to an unconjugated alkene. Nevertheless,
the pi electrons are still weakly nucleophilic as indicated by the red color
on the electrostatic potential surface. Thus benzene is still able to react
with wicked strong electrophiles, as described in the next chapter. |
|
|
 |
|
|
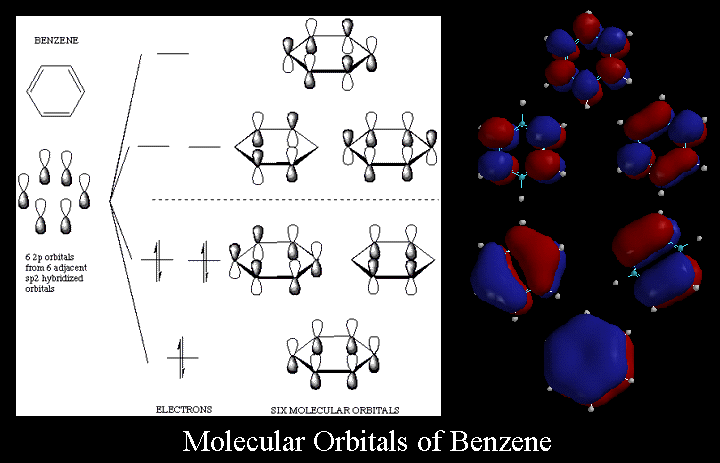
These
diagrams depict the molecular orbitals of benzene and illustrate why the
pi electrons in the benzene molecule are so unreactive compared to other
alkenes. When you look at the
molecular orbitals there are a number of things you should notice. First,
there are six molecular orbitals derived from the six 2p orbitals. Also there
are more nodes in the higher energy orbitals. Although we do not expect you
to remember the details of these orbitals, it is important you remember they
predict the important and surprising properties of benzene. For example,
the pi electrons are collectively 36 kcal/mole lower in energy than expected
for a molecule with 3 pi bonds, so the pi electrons of benzene are unreactive
towards the alkene addition reagents we are familar with, like Br2.
From the diagram you can see that the benzene molecule has a pi orbital system
composed of 2p orbitals that extends over all six ring carbon atoms. The
6 pi electrons are not localized in single pi bonds but when the wave amplitudes
combine in phase, they have 6 2p orbitals in which they can roam around the
ring freely. It is important to realize that this molecule does not have
alternating single and double bonds but it has a pi system that makes it
aromatic. To reiterate Huckel's Rule, it is a flat, monocyclic structure
in which each ring atom contains a 2p orbital with 4n+2 (in this case, 6)
pi electrons. |
|
| Aromatic
ions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
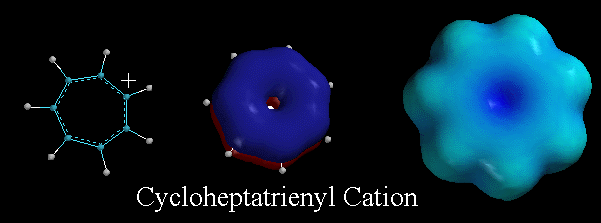
This
molecule, the cycloheptatrienyl cation (tropylium ion), also illustrates
that ions can be aromatic if they satisfy Huckel's Rule. That
is, ions can be flat, monocyclic compounds with 4n+2 electrons in their pi
system. This cation is very stable because the absence of electron density
is not localized in one 2p orbital but is delocalized over the entire pi
system. The blue color of the electrostatic potential surface diagram reiterates
this point. Note that all seven carbon atoms are equivalent, with a +1/7
charge on each carbon. |
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
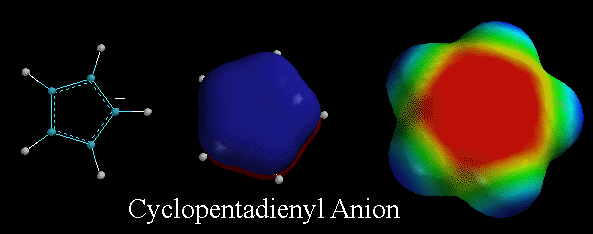
The
cyclopentadienyl anion is a very stable anion because it is aromatic. The
lone pair of electrons on the carbon atom are in a 2p orbital and are needed
to create the aromatic pi system as defined by Huckel. Therefore this lone
pair of electrons is highly delocalized, and is responsible for the stability
of the cyclopentadienyl anion. On the electrostatic potential surface, notice
how the area of high electron density (red color) is spread out over a large
part of the molecule and not just localized on one carbon atom. Also recall
that because this anion is so stable, the carbon atom on cyclopentadiene
is very acidic, or very easily gives up a hydrogen molecule to form this
aromatic system. Note how the cyclopentadienyl anion system is similar to
pyrrole , except pyrrole is neutral and the cyclopentadienyl anion has a
negative charge. |
|
|
|
|
|
|
|
|
Basicity
of Pyrindine v.s Pyrrole. Where is the lone pair? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
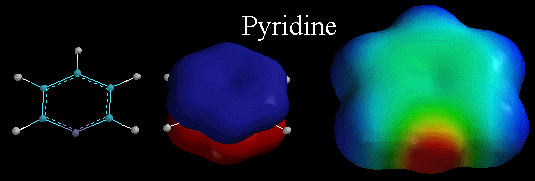
Above,
are three diagrams illustrating the characteristics of pyridine. Pyridine
is a flat, monocyclic compound with 6 pi electrons, making the molecule AROMATIC.
The picture in the middle is the lowest energy pi molecular orbital, emphasizing
that the pi electrons in this orbital are distributed over the entire ring.
Recall that for pyridine, the lone pair of electrons on the N atom are in
an sp2 orbital and are NOT part of the delocalized pi system. Therefore they
ARE localized and are available for binding to protons. Thus, the pyridine
is BASIC. You can see this by looking at the electrostatic potential surface
of pyridine on the right. Notice the localized area of electron density (the
deep red color) associated with the lone pair of electrons on the N atom. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
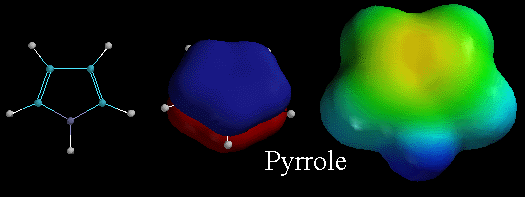
Like
pyridine, pyrrole is also an aromatic heterocyclic compound. The
middle picture shows the lowest energy pi molecular orbitals, emphasizing
that the pi electrons in this orbital are distributed over the entire ring.
Recall that for pyrrole, the lone pair of electrons on the N atom are in
a 2p orbital and are part of this delocalized pi system. Therefore, these
lone pair electrons are delocalized and are NOT available for binding to
protons. Thus pyrrole has NO area of localized electron density on nitrogen
(no deep red color as shown in the electrostatic potential surface on the
right) and so pyrrole is NOT VERY BASIC. Contrast this to the electrostatic
potential surface diagram for pyridine which has a localized area of electron
density. |
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |






