|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
 |
|
 |
|
 |
|
|
|
|
|
 |
|
 |
|
|
|
|
|
|
|
|
Pictures
of the Day 4-25-08
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amine
and Aniline Structure |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
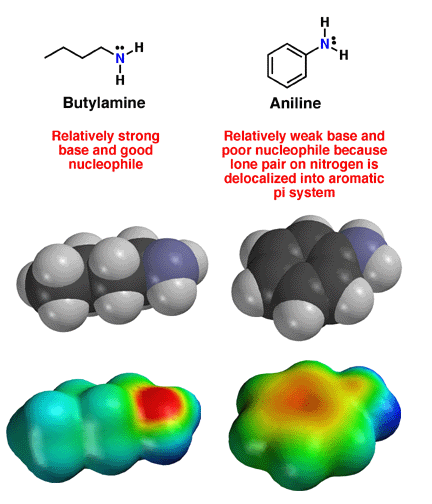
Above
are the structures of the butylamine and aniline. Note
how the amine nitrogen atom in butylamine has a localized lone pair of electrons.
This lone pair is available to interact with Lewis acids such as protons,
explaining the red color on the electrostatic potential surface, as well
as the fact that amines are good bases and strong nucleophiles. In aniline,
the nitrogen atom is planar and thus sp2 hybridized. This occurs because
the lone pair of electrons on the nitrogen is now in a 2p orbital, and thus
able to delocalize into the aromatic ring. Pi electrons prefere to delocalize
whenever possible, so this is a favorable interaction. Note how the electron
density of the lone pair, partially delocalized into the pi system of the
armatic ring, is less available to interact with protons. As a result, aniline
is a much weaker base and nucleophile compared
to alkyl amines. |
|
|
|
|
|
 |
|
|
|
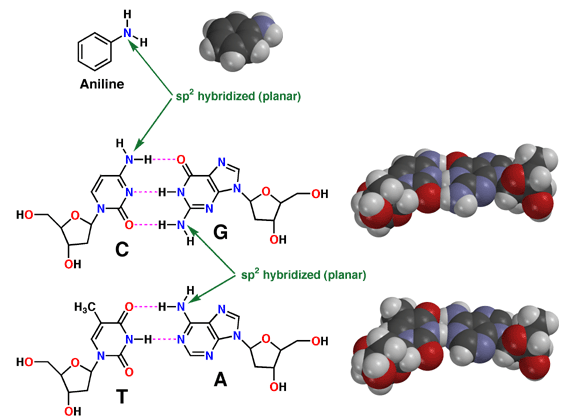
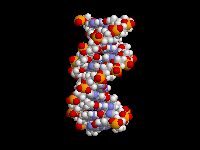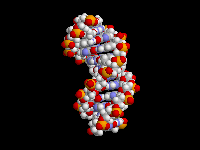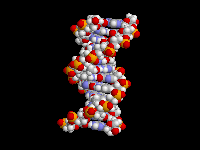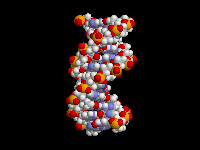
| The
fact that aromatic amines like aniline are planar and sp2 hybridized is extremely
important in biology. There are many biological molecules
with aromatic amines, such as the nucleic acid bases. Because these amine
groups are planar, the bases can hydrogen bond in the familiar Watson-Crick
base pairing arrangement as shown above. In addition, since the entire base
is planar, base-pairs can stack like a spiral staircase in the DNA double
helix. Thus, the sp2 hybridization of aromatic amines is responsible in large
part for one of the most important three-dimensional structures in all of
biology. |
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |



