
Pictures of the Day CH320M/CH328M
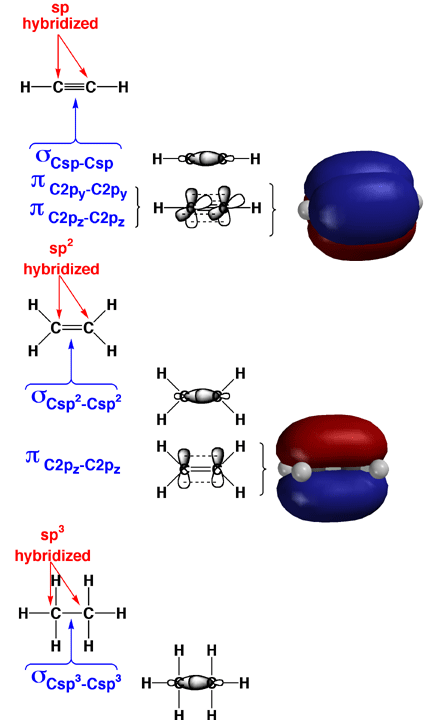
It is essential that you know how to describe bonding in organic molecules in the context of overlap of hybridized atomic orbitals, the so-called Valence Bond Approach. In the Valence Bond Approach, atomic orbitals are combined for each atom first (mathematically, this means that parentheses are drawn around the wavefunctions for each atom first), and it is the hybridized orbitals that overlap to make sigma bonds. In the molecules, any adjacent left over (non-hybridized) 2p orbitals are used to construct pi bonds. Hybridization state is assigned by looking at the Lewis structure for each atom (4 areas of electron density is sp3, three areas is sp2, and two areas is sp for C, N, O). Note that sigma bonds can rotate without being weakened, while pi bonds cannot rotate with being broken.
Above are shown how this is done for typical triple, double, and single bonds. In each case the sigma bonds are created from the overlap of hybridized atomic orbitals. Each sigma bond holds two electrons. The pi bonds are created from the overlap of unhybridized 2p orbitals. Each pi bond also holds two electrons. The pi bonds are shown as models to help you visualize the 2p orbital overlap and pi bonding.
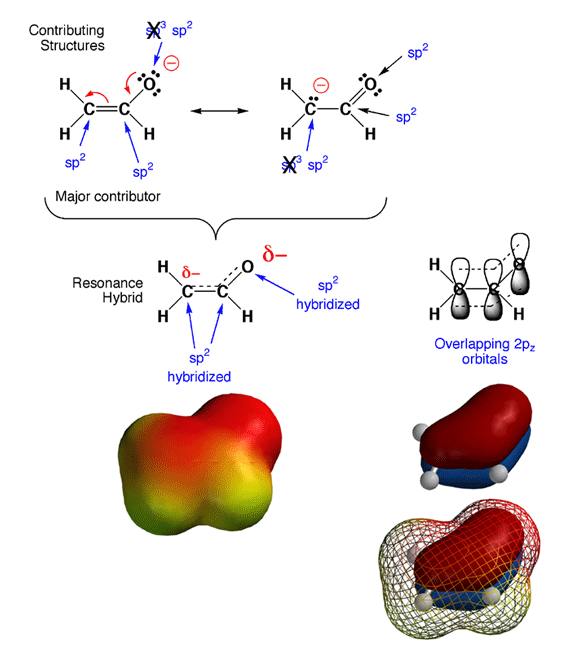
en you look back at your resonance contributing structures homework, you will notice something tricky. As in the case of the enolate ion shown above, the hybridization states for certain atoms are predicted to be different in different contributing structures. Since hybridization state does not really change, how do you decide which is the correct hybridization state? The answer is that in cases of resonance contributing structures in which a given atom is either sp3 or sp2 hybridized, the rule is that if any significant contributing structure indicates an atom is sp2, then sp2 is the hybridization state of the atom. In the example above, this means that the partially negatively charged carbon and oxygen atoms are both sp2 hybridized, even though they appear sp3 in one contributing structure each.
The reason for this "sp2 dominates" rule is that pi electrons prefer to be delocalized, and this can only occur if all the adjacent 2p orbitals overlap. In other words, molecules are more stable if pi electrons are delocalized over more than jsut two atoms. There are no 2p orbitals around an sp3 hybriduzed atom, so this stabilizing pi electron delocalization can only take place when the atoms are sp2 hybridized and the geometry is such that the 2p orbitals overlap. Note that with the electron delocalization in the pi orbitals, you also get charge delocalization, further stabilizing the overall structure. Thus, by adopting an sp2 geometry, the enolate ion is more stable (stabilization from both pi electron and charge delocalization), so sp2 is the hybridization that is observed. This is why the cases of resonance contributing structures you have seen involve the movement of electron pairs between pi bonds and lone pairs or between adjacent pi bonds. No single Lewis structure accurately conveys that the pi electrons are delocalized over more than two atoms, so we must draw contributing structures to make the point.