Click here for a PDF version of this handout
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED (HOPEFULLY)
Quantum Mechanics is a very difficult topic, with a great deal of detail that is extremely complex, yet interesting. However, in this Organic Chemistry Class we only need to understand certain key aspects of Quantum Mechanics as applied to electronic theory. What follows is an outline of many of the important concepts, color coded to help you. The statements in red are items you need to know. Items in black are for your information, but it is not essential that you know them. Items in purple describe what you need to be able to do, namely describe organic molecules in terms of overlap of hybridized orbitals. Keep this in mind as you go through the following.
QUANTUM OR WAVE MECHANICS
Electrons have certain properties
of particles and certain properties of waves.
Electrons have mass and charge like particles.
Because they are so small and are moving so fast, electrons have no defined position. Their location is best described by wave mechanics (i.e. a three-dimensional wave) and a wave equation called the Schrödinger equation.
Solutions of the Schrödinger equation are called wave functions and are represented by the Greek letter psi.
Each wave function describes a different orbital.
There are many solutions to the Schrödinger equation for a given atom.
The sign of the wave function can change from positive
(+) to negative (-) in different parts of the same orbital. This is analogous
to the way that waves can have positive or negative amplitudes.
The sign of the wave function does not
indicate anything about charge. [This can be confusing. Make sure that you
understand it before you go on.]
The value of the square of the wave function is proportional to the probability of finding electron density at a given point in an orbital. Note that the sign of square of the wave function is always positive, because the square of even a negative value is still positive.
In a 2p orbital, it is just as probable to find electron density in the negative lobe as it is to find electron density in the positive lobe. [Make sure you understand this statement.]
A node is any place in an orbital at which the value of the wave function is zero.
A nodal surface or nodal plane are surfaces or planes where the value of the wave function is zeor. There is absolutely no electron density at a node, a nodal surface, or a nodal plane.
-The Schrödinger equation can in principle describe covalent bonding, but, even with powerful computers the equation is too complicated to be solved exactly for large molecules.
MOLECULAR ORBITAL THEORY OF COVALENT BONDING
Molecular orbital theory assumes that individual electron pairs are found
in molecular orbitals that are distributed over the entire molecule.
Molecular orbitals are analogous to atomic orbitals and are described by the
following four rules:
First, combination of n atomic orbitals in a molecule or ion forms n molecular orbitals, each of which extends over the entire molecule or ion. The number of molecular orbitals is equal to the number of atomic orbitals combined, because atomic orbitals can be combined by both in phase and out of phase addition.
Second, molecular orbitals, just like atomic orbitals, are arranged in order of increasing energy.
Third, filling of molecular orbitals is governed by the same principles as the filling of atomic orbitals.
Electrons are placed in molecular orbitals starting with the lowest energy orbitals first.
A molecular orbital cannot hold more than two electrons.
Two electrons in the same molecular orbital have opposite spins.
When two or more degenerate (same energy) molecular orbitals are available, one electron is placed in each before any one of them gets two electrons.
When two atomic orbitals combine to form a molecular orbital, the wave functions
are combined both in phase and out of phase to create one bonding
molecular orbital and one antibonding molecular orbital, respectively.
A bonding molecular orbital occurs when the electron density of the orbital is concentrated between the atomic nuclei.
Electrons in bonding molecular orbitals stabilize covalent bonds because they serve to offset the repulsive forces of the positively-charged atomic nuclei. Both nuclei are attracted to the electrons between them.
The energy of a bonding molecular orbital is lower than the energy of the uncombined atomic orbitals.
An antibonding molecular orbital (designated with an *) occurs when the electron density of the orbital is concentrated in regions of space outside the area between the atomic nuclei.
Electrons in antibonding molecular orbitals do not stabilize covalent bonds because the electrons are not positioned to offset the repulsive forces of the positively charged atomic nuclei.
The energy of an antibonding molecular orbital is higher than the energy of the uncombined atomic orbitals.
A
sigma bond occurs when the majority of the
electron density is found on the bond axis. (Figs. 1.10, 1.17, 1.18
in text)
For example, a sigma bond results from the overlap between two 1s orbitals.
Because rotating sigma bond does not decrease the overlap of the orbitals involved ( sigma bonds have cylindrical symmetry), a sigma bond can rotate freely about the bond axis.
A pi bond occurs when the majority of the electron
density is found above and below the bond axis. (Fig.
1.21, 1.25 in text)
For example, a pi bond results from the overlap of two 2p orbitals that are parallel to each other, and orthogonal to the sigma bond that exists between the two atoms.
Because rotating a pi bond by 90° destroys the orbital overlap, pi bonds cannot rotate around the bond axis. [Understand this before going on.]
An electronic ground state occurs when all of the electrons are in the molecular
orbitals of lowest possible energy. An electronic excited state occurs when
an electron in a lower lying orbital is promoted to an orbital that is higher
in energy. This can occur when light is absorbed by a molecule, for example.
VALENCE BOND THEORY OF COVALENT BONDING
For elements more complicated than hydrogen, it is helpful to combine (hybridize)
the valence atomic orbitals on a given atom before looking for overlap with
orbitals from other atoms.
For C, N, and O hybridization means the 2s atomic orbital is combined with one, two, or all three 2p atomic orbitals.
The results of the orbital combinations are called hybrid orbitals, the number of hybrid orbitals are equal to the number of atomic orbitals combined.
An sp3 hybrid orbital is the combination of one 2s orbital with three 2p orbitals.
(Fig. 1.12 in text)
Four sp3 orbitals of equivalent energy are created.
Each sp3 orbital has one large lobe and a smaller one of opposite sign pointing in the opposite direction (with a node at the nucleus). The large lobes point to different corners of a tetrahedron (109.5° bond angle). This explains the tetrahedral structure of molecules like methane, CH4.
An sp2 hybrid orbital is the result of combining the 2s orbital with two 2p
orbitals. (Fig. 1.14 in text)
Three sp2 orbitals of equivalent energy are created.
Each sp2 orbital has one large lobe and a smaller one of opposite sign pointing in the opposite direction (with a node at the nucleus). The large lobes point to a different corner of a triangle (120° bond angle). This explains the trigonal planar structure of molecules like formaldehyde, CH2=O.
The left over 2p orbital lies perpendicular of the plane formed by the three sp2 orbitals.
An sp hybrid orbital is the combination of one 2s orbital with one 2p orbital.
(Fig.
1.16 in text)
Two sp orbitals of equivalent energy are created.
Each sp orbital has two lobes of opposite sign pointing in opposite directions (with a node at the nucleus). The lobes with like sign point in exactly opposite directions (180° bond angle). This explains the linear structure of molecules like acetylene.
The two left over 2p orbitals are orthogonal to each other, and orthogonal to the two sp hybrid orbitals as well.
Carbon atoms in molecules are either sp3, sp2, or
sp hybridized. 1s Orbitals are not considered for hybridization with C, N,
or O because the 1s orbitals do not participate in covalent bonding.
The hybridization of
a given atom (sp3, sp2, or sp) determines the geometry and type of bonds made
by that atom. The important parameters associated with each hybridization
state of carbon are listed in the following table:
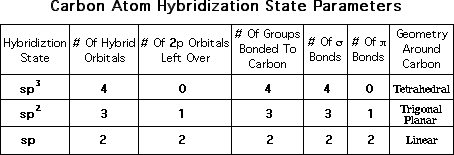
You should make similar tables for N and O
atoms.
**Bonding in complex molecules can be qualitatively
understood as overlap of hybrid orbitals.**
Organic chemistry is primarily concerned with
two types of covalent bonds, namely sigma (s)
bonds and pi bonds.
A sigma bond can be formed in a variety of ways.
(Figs. 1.10, 1.17, 1.18 in text)
A sigma bond results from the overlap between an s orbital and any other atomic orbital.
A sigma bond also results from the overlap of an sp3, sp2, or sp hybrid orbital and any s, sp3, sp2, or sp hybrid orbital along the bond axis. [You should be able to picture these different types of orbital overlap that all lead to s bonds.]
Because rotating a
sigma bond along the bond axis does not
decrease any orbital overlap, there is only a small barrier to rotation.
Thus, single bonds rotate extremely rapidly around the bond axis. This
explains why molecules with only
sigma bonds are highly flexible, able
to adopt an almost infinite number of rapidly interconverting conformations
in solution.
A double bond in molecules such as H2C=CH2
can be understood in terms of:
One sigma bond formed by sp2 hybridized orbitals on each carbon atom. (Figs. 1.18, 1.20 in text)
One pi bond formed between the 2p orbitals on each carbon atom. (Figs. 1.20, 1.21 in text and Picture)
Because rotating a pi bond by 90° destroys the orbital overlap, there is a large barrier to rotation and for all practical purposes double bonds cannot rotate around the bond axis. [Make sure this makes sense to you before moving on. The fact that pi bonds cannot rotate adds rigidity to molecules that contain them. This rigidity imparted by pi bonds has a major influence on the conformations of complex molecules in solution.]
A triple bond in molecules such as acetlyene can be understood in terms of:
One sigma bond formed by sp hybridized orbitals on each carbon atom. (Figs. 1.22 in text)
Two orthogonal pi bonds formed between the two orthogonal 2p orbitals on each carbon atom. Because rotating a pi bond by 90° destroys the orbital overlap, triple bonds also cannot rotate around the bond axis. (Figs. 1.22 in text and Picture)
[It is absolutely essential that students understand bonding in complex molecules in terms of the overlap of hybrid orbitals. This orbital picture of molecules forms the theoretical foundation for understanding the reactions of the different functional groups discussed in the rest of the semester. Do not go any further until these concepts are thoroughly understood.]
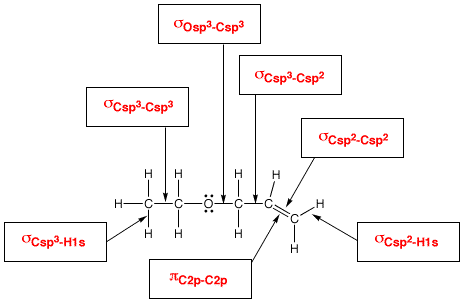
For example, below we show how we want you to think about the electronic structure of molecules according to the valence bond approach.
In the boxes are shown the hybridization state of selected atoms. You should be able to assign the correct hybridization state of all atoms in organic molecules.