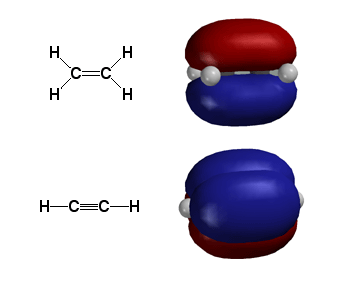
Upper Molecule: The pi bonding orbital of ethylene ("hot dog bun"), formed from the overlap of the 2p atomic orbitals on each carbon atom (gray atom in figure). The sigma bonding orbital ("hot dog meat"), formed from overlap of sp2 hybridized orbitals on each carbon atom, is NOT shown.
Lower Molecule: The pi bonding orbitals of acetylene ("two hot dog buns"), formed from the overlap of both 2p atomic orbitals on each carbon atom (gray atom in figure). The sigma bonding orbital ("hot dog meat"), formed from overlap of sp hybridized orbitals on each carbon atom, is NOT shown. The two pi bonds are orthogonal to each other, because the two 2p orbitals on each carbon atom are orthogonal.
Click here to go back to web handout on molecular orbital and valence bond theory.