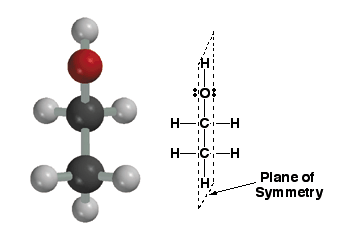
|
This
molecule, ethanol, is achiral (not chiral) because it posesses a plane
of symmetry. A plane of symmetry is a plane that cuts through a molecule
such that one half of the molecule is a perfect reflection of the other
half of the molecule. The plane of symmetry in this molecule is represented
by the dashed line. Note that this molecule also does not have a chiral
center. Almost (but NOT always), a molecule needs a chiral center to
be chiral. Remember, when looking for planes of symmetry, you are allowed
to choose the most symmetric conformation and temporarily ignore the
concepts you have learned about conformational preference. Don't worry
about relative conformational energy when looking for symmetery. Because
enthanol is not chiral, its mirror image is not a different stereoisomer,
rather it is the same molecule (therefore its mirror image is superimposable
on itself). Click
here to go back to stereochemistry web handout. |