STEREOCHEMISTRY
HOPEFULLY MADE SIMPLER
Stereoisomers-molecules that have the same constitution, but different disposition
of groups in space. In
other words the atoms are connected to each other in the same way,
they only differ with respect to relative orientation in three--dimensional
space.
Chiral-General Definition-Any
object that is not superimposable on its mirror image.
Your hands are chiral, that is why you need two different leather
gloves, one that only fits your right hand, and one that only fits your
left hand. If your hands were superimposable, then you would only need
one kind of glove and it would fit both hands.
Chiral-Chemistry Definition;
Atom-Any tetrahedral carbon atom that has four different
substituents is a chiral center. Any tetrahedral carbon atom that has
four different substituents is a chiral center
(it was worth repeating). This is a simple consequence of geometry;
there are two
different ways to
place four different substituents in a tetrahedral arrangement.
Looking for four different substituents on a single carbon atom
is the easiest way to identify a chiral molecule. It
is worth mentioning that a chiral center is a special type of a more general
situation called a stereocenter. A stereocenter is any atom
in a molecule for which exchanging two groups creates a different
stereoisomer. All chiral centers are stereocenters, however, not
all stereocenters are chiral centers as we will encounter examples of this
in later chapters. Do not sweat this detail at this point. The bottom line
is that when you have a tetrahedral carbon atom with four different groups,
call it a chiral center and life will be good.
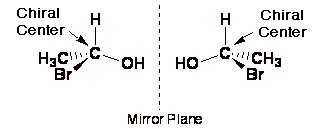
As a further consequence of simple geometry, there are only two different ways to place four substituents around a chiral center, and as shown above the resulting two different molecules are mirror images of each other. They are non-superimposable mirror images of each other. You should make models of the above molecules and prove to yourself they are different and non-superimposable if you haven't done this already. This pair of chiral molecules that are mirror images of each other are called enantiomers, a chemistry name given to represent this special mirror image relationship between different molecules.
A carbon atom is not a chiral center if even two of the substituents are the same. A carbon atom is not a chiral center if even two of the substituents are the same (that was also worth repeating). Historical note: in the past, chiral centers have also been called; stereogenic centers, asymmetric carbon atoms, asymmetric centers, or chiral atoms even though these names can be confusing in certain situations.
Chiral-Chemistry
Definition; Molecule-
A molecule is not chiral (even if it has chiral centers) if it has
a plane or center of symmetry. A
plane of symmetry
is any plane cutting through the molecule such that one side is a perfect
reflection of the other. When looking for a plane of symmetry you have to
put the molecule in the most symmetric conformation possible, DO
NOT WORRY ABOUT WHICH CONFORMATION IS MOST STABLE, STABILITY OF
CONFORMATION IS NOT IMPORTANT FOR STEREOCHEMISTRY. This means
you look for symmetry in cyclohexane derivatives that are drawn
flat and alkanes that are eclipsed !
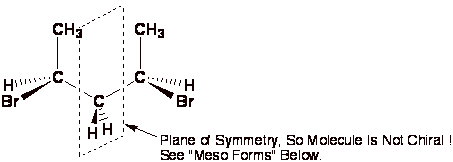
A center of symmetry
is any point is space such that any group on the molecule can be reflected
back through that point an equal distance but opposite direction and an
equivalent group is found. This is relatively rare in organic chemistry.
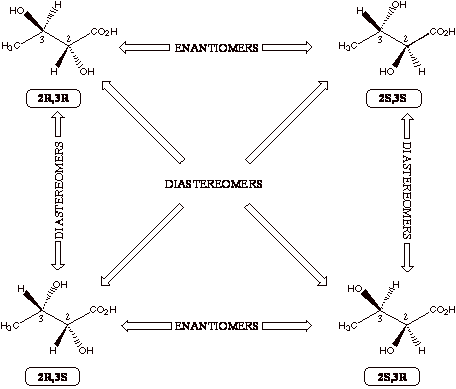
Molecules With More Than One
Chiral Center If
there are two chiral centers in a single molecule, there are four
possible stereoisomers. This
is because each carbon atom can be in one of two possible forms
(R or S) as we saw above, so there are 2x2=4 possible combinations.
Now refer to the general figure below as well as the figure
on the following page with the four chemical structures. Make sure you understand all the relationships of the four
different molecules shown. It
is easiest to remember that enantiomers must be mirror images
of each other. Diastereomers
is the chemistry term that describes the relationship beween the pairs of molecules in the figures that are
not even mirror images of each other.
In other words, each pair of molecules in the figures must be
related because they are all stereoisomers of each other, so they
are either enantiomers (mirror images) or diastereomers (not mirror
images). Thus, diastereomers are stereoisomers
that are not enantiomers. If a molecule happens to
be symmetric so that two of the four possible stereoisomers are identical
(the S,R is identical to the R,S; the S,S and R,R will always be
enantiomers), this form of the molecule is called the meso
form. Since
this situation requires some special symmetry (usually a plane of
symmetry) to be present, it is the unusual exception, not the rule.
To Determine Whether Molecules Are Enantiomers, Diastereomers
or Meso Compounds On A Test: *
1). Determine the absolute (R or S) stereochemistry
at each chiral center. Click here
to go to a description of how to determine whether a chiral center
is "R"
or "S".
2). Use the following table to determine relationship.
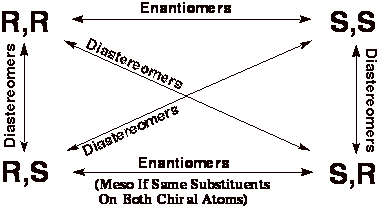
3). If
the molecule happens to be symmetric, check for meso compound, remembering
that only the R,S or S,R molecules can be meso (the S,S and R,R forms of
even symmetric molecules are not meso compounds, they are enantiomers).
* This method is much easier than simply trying to look
for mirror images etc. by rotating molecule in your mind or redrawing structures
in various orientations.
Other Important Ideas:
1). Different enantiomers can only be distinguished
by chiral things (optical activity etc.). Living
systems and biological molecules are chiral, so chiral molecules are almost
always distinguished by living systems.
2). Different diastereomers can be distinguished
by all of their chemical properties.
3). A racemic mixture is defined as being
a one-to-one mixture of two enantiomers.