|
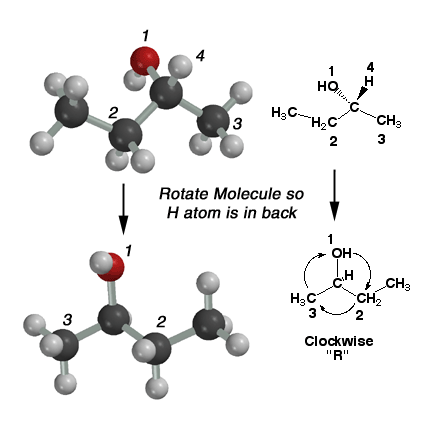
2-Butanol
can exist as two possible stereoisomers. More specifically, the two stereoisomers
are enantiomers of each other. Because they are stereoisomers, they
are different molecules and it is necessary to distinguish the two
molecules using the "R" and "S" nomenclature system. In order to designate
a chiral center as "R" or "S", we must label the substituents around
the chiral carbon atom in order of increasing priority. We use
the rules a) atomic weight wins, b) look for the FIRST point of difference
(this is most student's source of error) and c) count multiple bonds
as just more single bonds (look in the book for a better description
of this). In the case of 2-butanol above, the hydroxyl group is most "important" and
labeled #1, and the hydrogen atom is least "important" and labeled
#4. Next is the ethyl group (#2) followed by the methyl group (#4).
The molecule is rotated so the lowest ranking group (#4, the H atom)
is pointed away from you. Count the numbers in order #1 - #2 - #3.
This motion is clockwise in the above molecule, therefore this is R-2-butanol.
If the direction had been counterclockwise, it would have been S-2-butanol. Click
here to go back to stereochemistry web handout. |