|
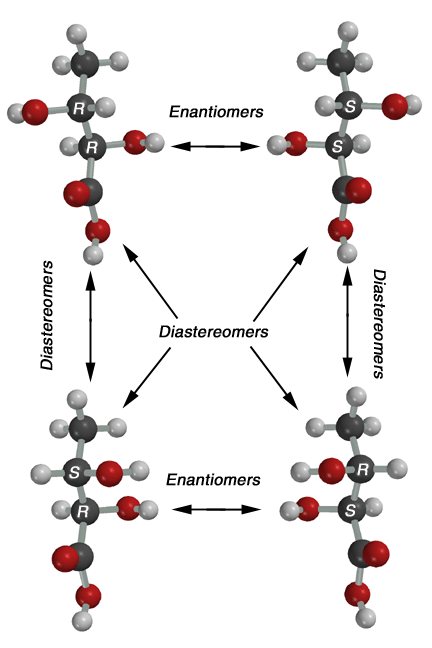
These
structures match the structures in the back of the web
handout on stereochemistry, so you should refer to that. This molecule,
2,3-dihydroxybutanoic acid, has two stereocenters (one at carbon 2, and
one at carbon 3, so there are four possible stereoisomers. The 2R,3R and
2S,3S stereoisomers are enantiomers of each other, and the 2R,3S and 2S,3R
are enantiomers of each other. These are enantiomer pairs becuase they are
nonsuperimposable mirror images of each other. Any other pair, such as 2R,3R
and 2S,3R, are diastereomers of each other, since they are stereoisomers
that are not enantiomers (not mirror images of each other). Thus, you use
the terms "enantiomers" or "diastereomers" to describe
the stereochemical relationship when comparing different stereoisomers |