Click here for a printer friendly pdf version of all of the Golden Rules pages
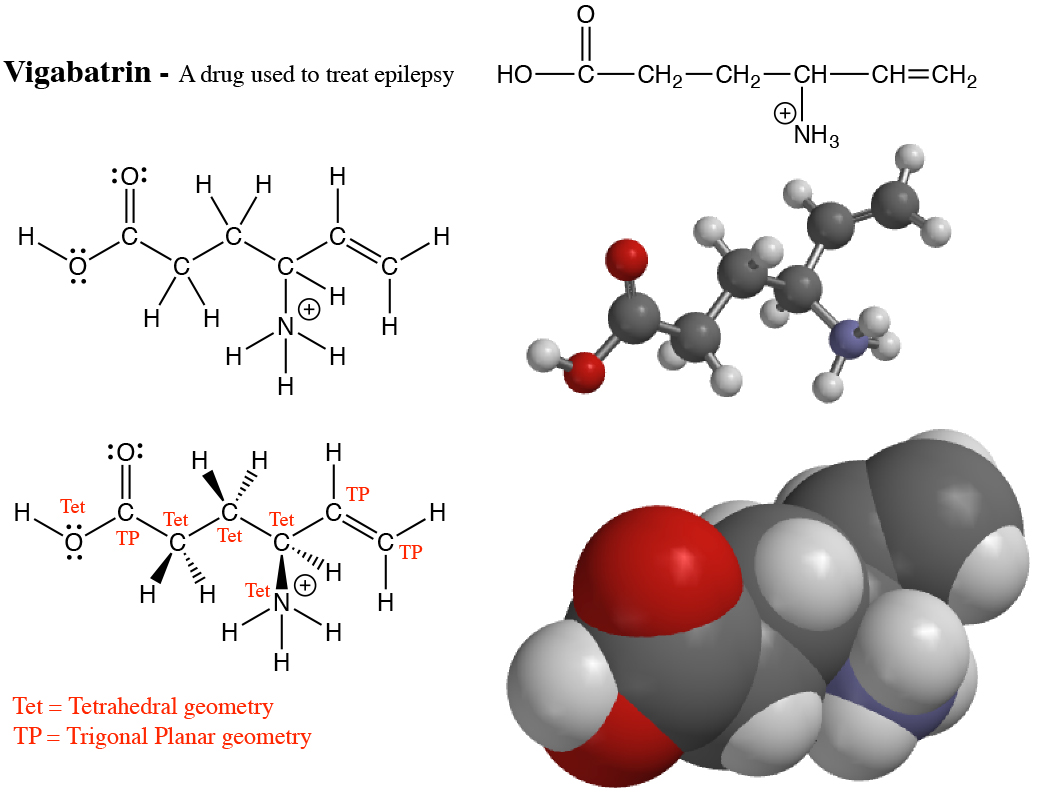
1. In most stable molecules, all the atoms will have filled valence shells. This means that C, N, O and the halogens will have 8 electrons in their valence shells, and H atoms will have 2 electrons in their valence shells. This simple yet powerful principle predicts the type of bonds created (single, double or triple) and how many lone pairs are found around the different atoms of a molecule. In general, an atom surrounded by 4 atoms/lone pairs will have a tetrahedral geometry, an atom surrounded by 3 atoms/lone pairs will have a trigonal planar geometry and an atom surrounded by two atoms/lone pairs will have a linear geometry. You will encounter a small number of molecules containing an atom such as a C atom with only 6 or 7 electrons in its valence shell. Atoms such as this with only a partially filled valence shell are noteworthy and highly reactive. Note, however, that you can never overfill the valence shell of any atom in a molecule such as placing more than 8 electrons in the valence shells of C, N, or O.