Click here for a printer friendly pdf version of all of the Golden Rules pages
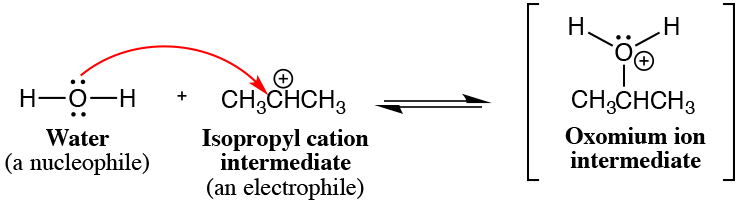
11. Most bond-making steps in reaction mechanisms involve nucleophiles reacting with electrophiles. Nucleophiles are molecules that have a lone pair or bond that can donate electrons to make a new covalent bond, usually corresponding to an area of relatively high electron density. Electrophiles contain atoms that can accept the new covalent bond, usually corresponding to areas of relatively low electron density or even an unfilled valence shell. Note that often a covalent bond is broken in the electrophile to make room for the new covalent bond being made (but not in the example below).