Click here for a printer friendly pdf version of all of the Golden Rules pages
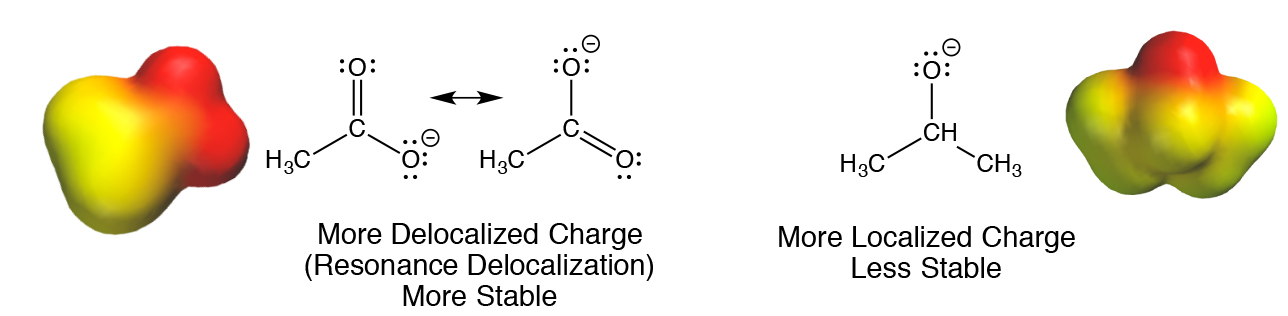
5. Delocalization of charge over a larger area is stabilizing. The majority of molecules you will encounter will be neutral, but some carry negative or positive charges because they contain an imbalance in their total number of electrons and protons. In general, charges are destabilizing (higher Gibbs free energy), increasing the reactivity of the molecules that possess them. Localized charges are the most destabilizing (highest Gibbs free energy). Delocalizing the charge over a larger area through interactions such as resonance, inductive effects, and hyperconjugation is stabilizing (lowering the Gibbs free energy). In addition, it is more stabilizing to have more negative charge on a more electronegative atom (e.g. O), and more positive charge on a less electronegative atom (e.g. C).