Click here for a printer friendly pdf version of all of the Golden Rules pages
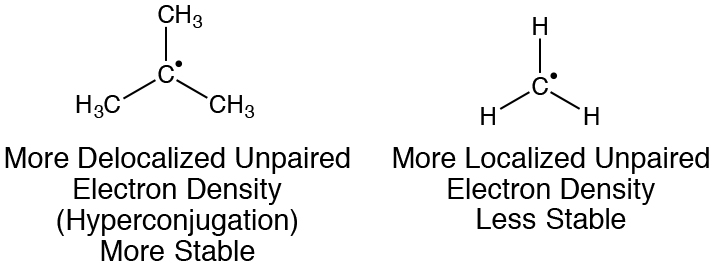
6. Delocalization of unpaired electron density over a larger area is stabilizing. The majority of molecules you will encounter will only have atoms with filled valence shells and therefore an even number of electrons. These electrons will have paired spins. However, especially in Chapter 8, you will encounter some molecules with an odd number of valence electrons and therefore an unpaired electron. In general, unpaired electron density is destabilizing (higher Gibbs free energy), dramatically increasing the reactivity of the molecules that possess it. Highly localized unpaired electron density is the most destabilizing (highest Gibbs free energy). Delocalizing the unpaired electron density over a larger area through interactions such as resonance and hyperconjugation is stabilizing (lowering the Gibbs free energy).