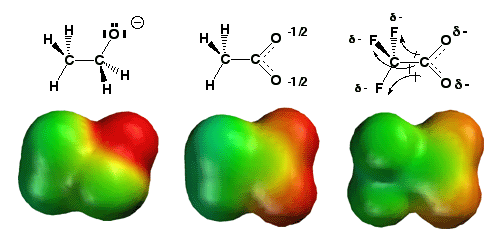Pictures of the Day CH320M/CH328M
9-29-29
Relative
Anion Stability - Resonance and Inductive Effects
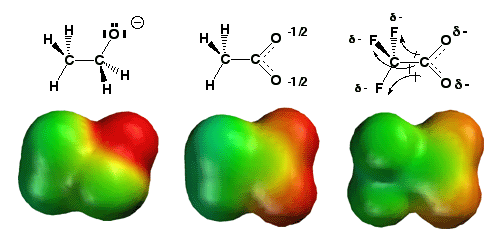
A
good way to predict relative acid strength is to look at the conjugate
base anions produced upon deprotonation of a set of acids, and predict
which anion will be the most stable anion. The more stable the conjugate
base anion, the more acidic the parent acid. The
best way to stabilize an anion is to spread the negative charge around.
Shown above on the left is the ethoxide anion (CH3CH2O-),
with the negative charge (darkest red color) localized entirely on the
oxygen atom. The molecule in the middle is the acetate anion (CH3CO2-),
and notice how the negative charge (lighter red color) is distributed
over the two oxygen atoms, as explained by the two relevant resonance
structures you should be able to draw for this species. Spreading the
charge due to this so-called "resonance effect" is extremely
stabilizing compared to the ethoxide anion so it should be no surprise
that the parent acid of the acetate anion, namely acetic acid, has a
pKa of 4.75 and is significantly more acidic than the parent acid of
ethoxide anion, namely ethanol, having a pKa of around 16. The molecule
on the right is the trifluoroacetate anion (CF3CO2-),
and here, like the acetate anion in the middle, there is a resonance
effect helping to distribute the negative charge over the two oxygen
atoms. However, with the trifluoroacetate anion, there are also three
electronegative fluorine atoms attached nearby, and these pull some electron
density through the sigma bonding network away from the oxygen atoms,
thereby spreading out the negative charge (lightest red color) even further.
This effect, called the "inductive effect" is stabilizing to
anions such as the trifouoroacetate anion so the parent acid, trifluoroacetic
acid is the strongest acid of the three by far, having a pKa of -0.18.