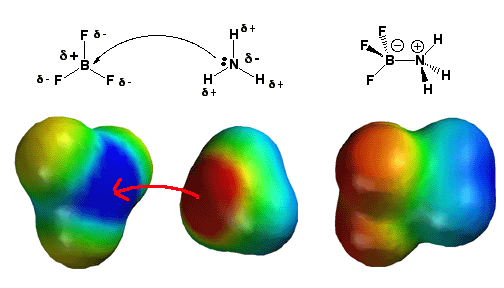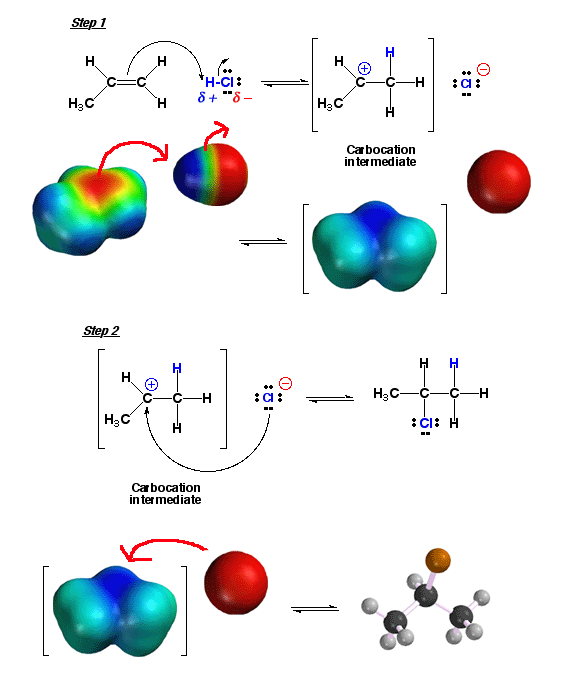Alkenes
- Its All About the Pi Bond!
Pictures of the Day CH320M/CH328M
10-1-25
Lewis
Acids and Bases
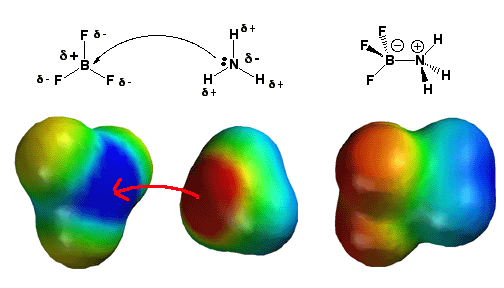
Shown
on the left is the BF3 molecule. With
three bonds to fluorine (sp2 hybridization), and no lone pairs, there
remains one 2p-orbital that is not hybridized and empty. Thus the boron
atom needs a lone pair of electrons to give it an octet of electrons.
Also, fluorine is highly electronegative, withdrawing electron density
from the boron atom. This is represented in the electrostatic potential
model at the upper-left, with flourine atoms in yellow-orange (partial
negative charge) and boron in blue (partial positive charge). For all
these reasons, the molecule BF3 is a good acceptor
of electrons and therefore a good Lewis acid. Ammonia (NH3), shown
in the middle, has a lone pair of electrons, and since nitrogen
is more electronegative than hydrogen, the nitrogen atom has a partial
negative charge (red color). In this diagram, NH3 acts
as a Lewis base,when it donates its lone pair of electrons to BF3.
BF3 acts as a Lewis acid when it accepts the lone
pair of electrons that NH3 donates. This reaction
fills BF3's empty 2p-orbital, and now boron is
sp3 hybridized when previously (as BF3) it was
sp2 hybridized. Note the negative charge on the boron half of the molecule
(red color) and positive charge on the ammonia half of the molecule (blue
color).
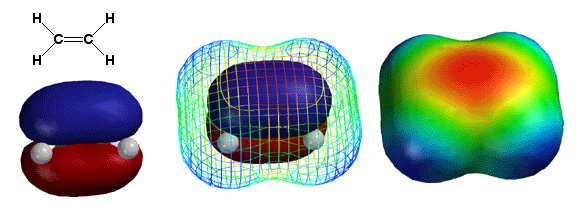
The
key to understanding alkene chemistry is keeping in mind the special
properties of the pi bond (the
pi bonding orbital is shown on the left). The electrons in the pi bond
are above and below the plane of the bond, so rotating the bond would
break it. Thus, as opposed to sigma bonds, pi bonds cannot rotate. This
makes possible cis and trans (E and Z) stereoisomers. In addition, the
electron density of the pi bond carries with it negative charge, meaning
that there is a partial negative charge (red color) above and below the
bond. This partial negative charge can interact with molecules having
areas of partial or full positive charge. This scenario, in fact, describes
almost all of the reactions of pi bonds we will see next chapter.
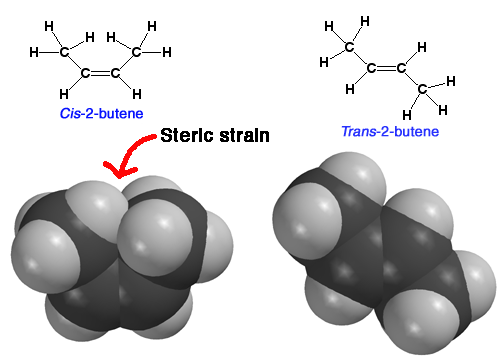
Relative
Alkene Stability - cis and trans
For
similar alkenes, a trans alkene will be lower in energy (more
stable) than a cis alkene. This is because a cis alkene
has some steric strain that the trans alkene does not have. Note
that more substituted alkenes are also more stable than less substituted
alkenes, but we do not really explain this. You have to just learn it.
H-Cl
Addition - Keep Track of the Partial Charges!
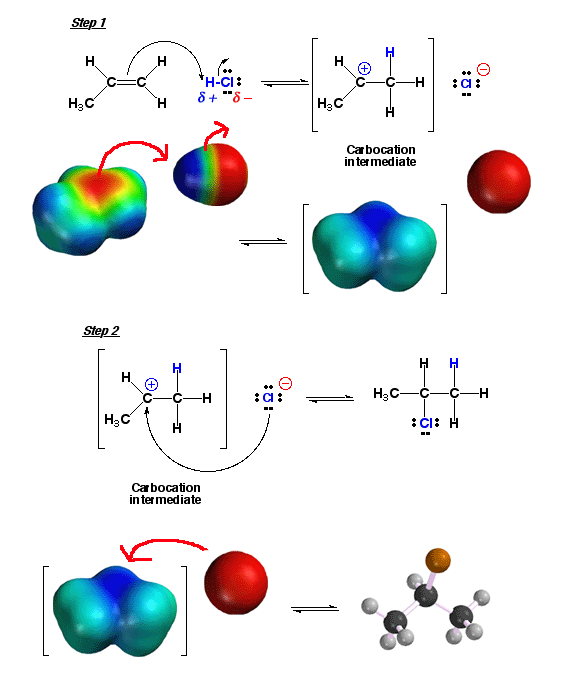
Most reaction mechanisms we will encounter involve the
four common mechanistic elements introduced in class. When
drawing mechanisms, always use arrows
to indicate the flow of electron density from the electron source, to the
electron sink atom. Try to predict which of the mechanistic elements
are going to occur under the reaction conditions. In
this way you will be learning and understanding the mechanisms (this
is good) not simply trying to memorize them (this is bad). If you understand
them, you will see how most mechanisms are related, and you will only
have to learn a few things before you can successfully write down correct
mechanisms (this is good).
For example, the addition of H-Cl can best be understood as "add a proton" mechanistic element followed by "make
a bond" between a nucleophile (Cl-) and an electrophile (the carbocation
intermediate) .
In the first step, electron density of the pi bond attacks the electrophilic
hydrogen atom of H-Cl to give a carbocation intermediate and the chloride anion.
Note how in the above reaction the more stable of the possible carbocations
is produced predominantly, namely the secondary (2°)
carbocation (thus explaining Markovinikov’s rule). In the next step, the
nucleophilic chloride anion attacks the carbocation electrophile to give the
final product. The nucleophile attack can come from the top or the bottom of
the trigonal planar (sp2 hybridized) carbocation with equal probability, no
matter which face of the alkene the H atom added to, so there is no stereochemical
preference of product produced. Click
here to see a movie of a related reaction, the addition reaction of H-Br with
an alkene.