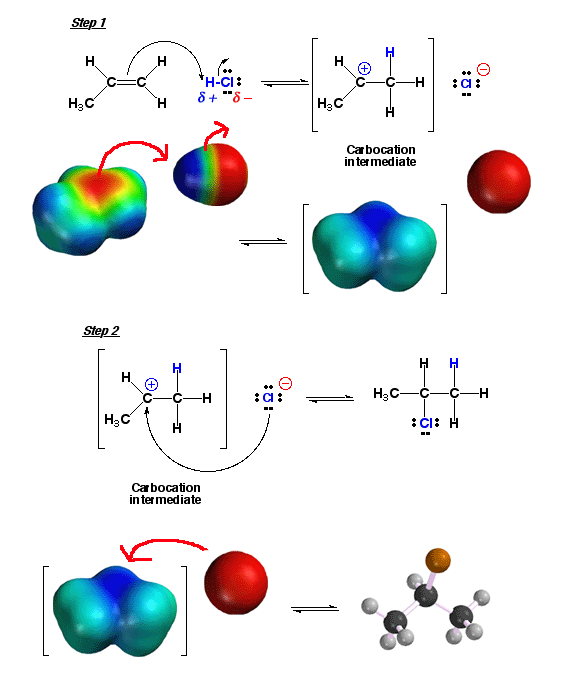Pictures of the Day CH320M/CH328M
10-6-25
H-Cl
Addition - Keep Track of the Partial Charges!
Most reaction mechanisms we will encounter involve the
four common mechanistic elements introduced in class. When
drawing mechanisms, always use arrows
to indicate the flow of electron density from the electron source, to the
electron sink atom. Try to predict which of the mechanistic elements
are going to occur under the reaction conditions. In
this way you will be learning and understanding the mechanisms (this
is good) not simply trying to memorize them (this is bad). If you understand
them, you will see how most mechanisms are related, and you will only
have to learn a few things before you can successfully write down correct
mechanisms (this is good).
For example, the addition of H-Cl can best be understood as "add a proton" mechanistic element followed by "make
a bond" between a nucleophile (Cl-) and an electrophile (the carbocation
intermediate) .
In the first step, electron density of the pi bond attacks the electrophilic
hydrogen atom of H-Cl to give a carbocation intermediate and the chloride anion.
Note how in the above reaction the more stable of the possible carbocations
is produced predominantly, namely the secondary (2°)
carbocation (thus explaining Markovinikov’s rule). In the next step, the
nucleophilic chloride anion attacks the carbocation electrophile to give the
final product. The nucleophile attack can come from the top or the bottom of
the trigonal planar (sp2 hybridized) carbocation with equal probability, no
matter which face of the alkene the H atom added to, so there is no stereochemical
preference of product produced. Click
here to see a movie of a related reaction, the addition reaction of H-Br with
an alkene.
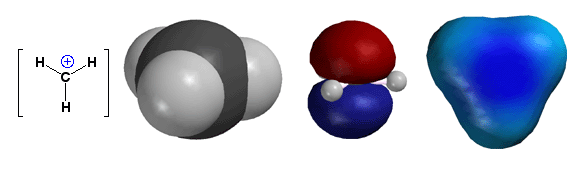
Carbocation
Structure - sp2 with an Empty 2p
Carbocation
hybridization is sp2, with an empty 2p orbital
(as shown on the right). Thus, carbocations are trigonal planar, a
fact that is important because it means carbocations can be attacked
by nucleophiles from the “top” or the “bottom” with
equal probability, leading to enantiomer products if a new stereocenter
is created. The nucleophiles start reacting with carbocations by placing
electron density (i.e. a lone pair of electrons) into the empty 2p
orbital. Carbocations are extremely reactive because
they possess both an unfilled valence shell and a full positive charge.
Thus, you will only encounter them as relatively high energy reaction
intermediates during the mechanisms of reactions.
Carbocation
Stability
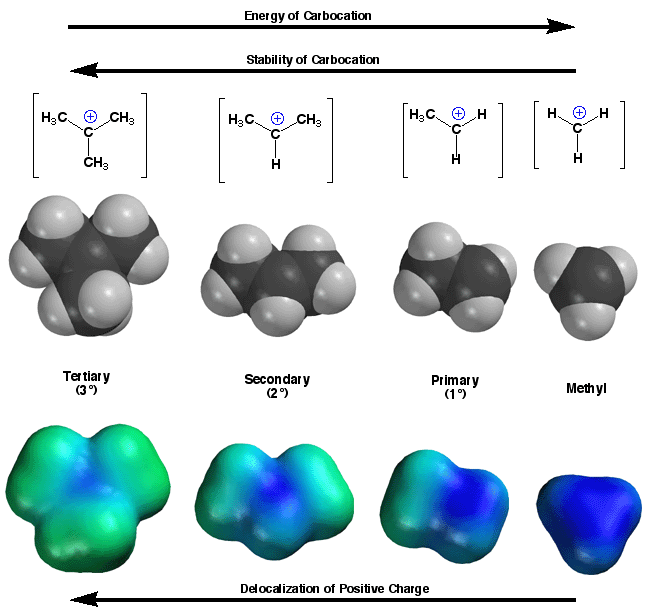
Carbocations
are stabilized by adjacent alkyl groups, so
more highly substituted carbocations are more stable. Alkyl groups stabilize
carbocations by a combination of an inductive effect (positively-charged
carbon is electronegative, i.e. it wants electron density, so it withdraws
some of the electron density of an alkyl group through sigma bonds) and
hyperconjugation in which the empty 2p orbital partially overlaps in
space with the C-H sigma bonds of the adjacent alkyl groups. In both
cases, some electron density is placed on the positively-charged carbon
atom from the alkyl groups, leading to a greater distribution of the
positive charge around the molecule. The more alkyl groups, the more
highly distributed is the positive charge. The
more highly distributed the positive charge, the more stable the carbocation.
You can see the dramatic differences in charge distribution quantitatively
on the bottom row in which carbocations with more alkyl groups attached
have less intense blue color on the positively-charged carbon atom, indicating
more delocalization of positive charge and thus greater stability. Keep
in mind that even tertiary (3°) carbocations are still carbocations
and extremely reactive with even weak nucleophiles, only surviving for
exceedingly short amounts of time during reaction mechanisms.