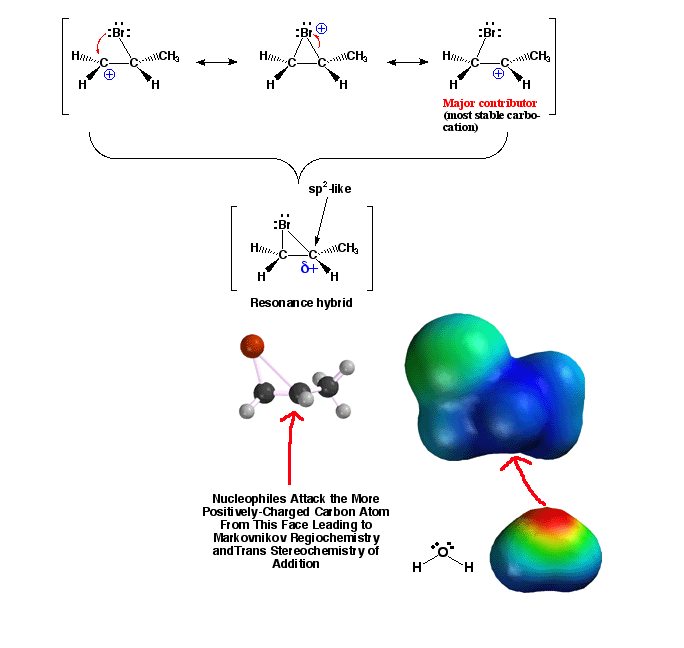
Substituted
Halonium Ions - Leads to Markovnikov Addition
Substituted
halonium ions have a greater amount of positive charge (more blue color)
on the carbon atom with the larger number of alkyl substituents. This
means that nucleophiles will attack at this position, explaining the
regiochemistry of the halohydrin reaction (the OH group ends up on the
more substituted carbon atom). The easiest way to understand this is
to think about the halonium ion as a resonance hybrid as shown. Since
the holonium ion requires attack from the side opposite the X atom, anti
addition stereochemistry is always observed, leading to trans products.
Pictures of the Day CH320M/CH328M
10-15-25