Alkynes
- 2 Pi Bonds Instead of One
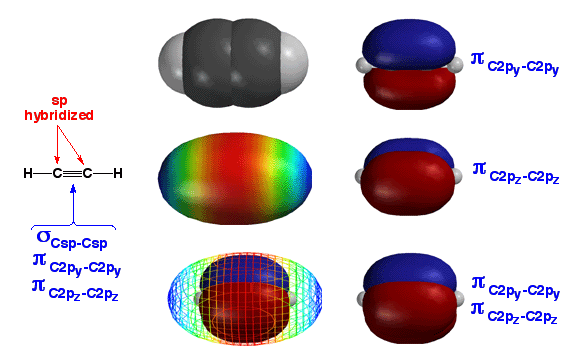
Above
are various representations of the simplist alkyne,
acetylene. The main point is that, like alkenes, the reactivity
of alkynes is dictated by the pi bonds. Due to the pi bonds, alkynes react
as weak nucleophiles with various electrophiles. Of course, alkynes react
two times with most electrophiles, since there are two pi bonds. A key difference
between alkynes and alkenes is that when water adds (H-O-H adds), alkynes
initially give enols, that spontaneously tautomerize to the keto form (giving
ketone or aldehyde final products).
Pictures of the Day CH320M/CH328M
10-28-24