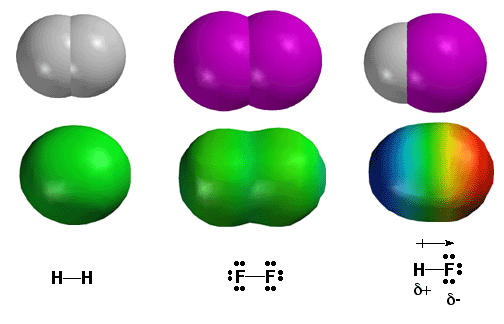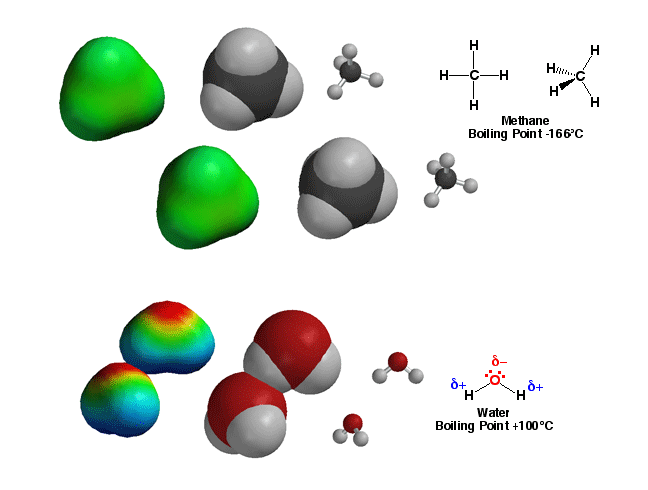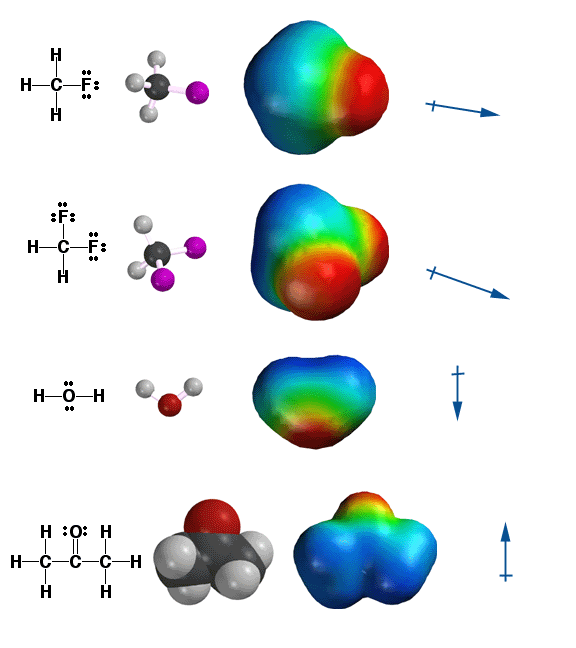8-27-25
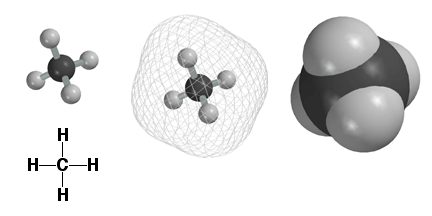
Atoms
are surrounded by electrons and we use the computer to plot molecules to
reflect where the electrons are. Above are three different computer images
of the methane molecule CH4. The image on the upper left shows the C and
H atoms as small balls connected by sticks, i.e. the bonds. This is a nice
picture, but does not really show where the electrons are, since they are
found farther away from the atomic nuclei. Shown in the middle above is a
mesh surface under which the electrons can be found. On the upper right is
shown a solid surface, color coded by atom type. This type of representation
is referred to as a space filling model. The space filling models are a better
way to think about molecules, since they give a realistic picture of molecular
size, electrons and all. Refer
to the atom type chart to remind yourself which atoms have which colors.
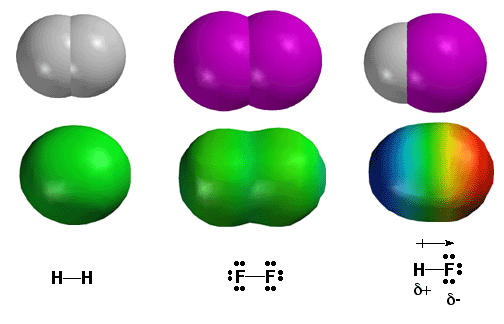
The
most important concept in chemistry is that
some atoms are more electronegative than others, in other words, they “hog” more
of the electrons in a covalent bond. Thus, electronegativity
answers the most important question in chemistry. Electron
density distribution in a molecule can be quantitatively displayed in
color coding using so-called electrostatic potential surfaces calculated
by computer. Shown above are three simple diatomic molecules. On the
left is H2, with a single bond between the H atoms. Since two H atoms
have the same electronegativity, they share the electrons evenly. This
is depicted as a green color on the electrostatic potential surface plot.
In the middle is the F2 molecule. Again, since both the F atoms of the
bond have the same electronegativity, the electron density is shared
evenly between the two atoms and the electrostatic potential plot is
green. On the right is the H-F molecule. Fluorine is much more electronegative
than hydrogen, so the majority of electron density resides on the fluorine
atom. High electron density around the F atom is depicted as the red
color on the electrostatic potential surface above. The hydrogen atom
has less electron density, depicted as blue color to indicate the absence
of electron density. This type of covalent bond, with the electrons shared
unevenly is called a polar covalent bond (difference in electronegativities
of bonded atoms is greater than 0.5). The polarity of the bond is quantified
by using the vector quantity of dipole moment, represented by the arrow
symbol above the bond. Note that since electron density has negative
charge, the fluorine atom has a partial negative charge (d-)
leaving a partial positive charge (d+) on
hydrogen.
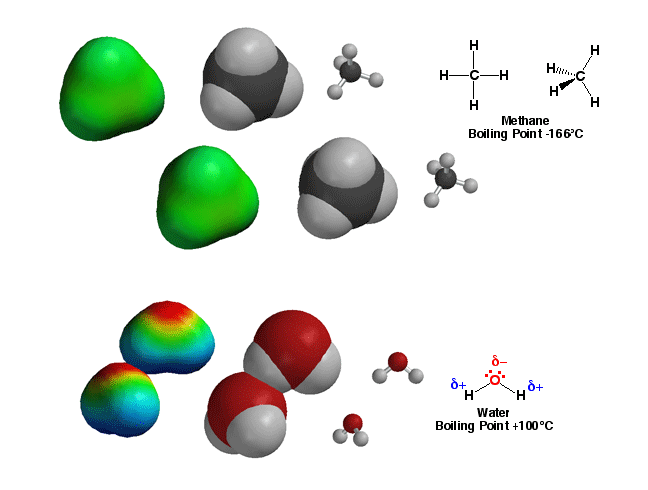
The polarity
of bonds in molecules predicts the properties and reactions of molecules.
For example, methane, CH4, has only non-polar
bonds. As a result, there are no significant partial charges in the
molecule, so molecules in a sample of methane interact only weakly. With
only very weak interactions, not very much energy is required to dissociate
the molecules (turn the sample into a gas), so the boiling point of methane
is very low, -166°C. On the other hand, water has very
polar O-H bonds, with the majority of bonding electron density residing
on the O atom. This electron distibution puts a partial negative charge on
the O atom, and partial positive charges on the H atoms. The result is that
the molecules are attracted to each other (opposite charges attract) with
the H and O atoms from different molecules in contact with each other as
shown above. This interaction, referred to as hydrogen bonding, means that
the molecules in a sample of water stick together relatively well, so a great
deal of energy is required to dissociate the molecules (turn to steam). At
100°C, the boiling point of water is 266°C higher than that of methane!
This huge difference in properties for these two molecules of the same size
can only be understood in the context of bond polarity, namely the presence
of partial positive and negative charges, which itself is simply knowing
where the electrons are! You will learn how reactions of molecules will also
be predictable by knowing which bonds are polar, since these are usually
the reactive bonds in organic molecules.
BOND ANGLES AND SHAPES
OF MOLECULES
• The Valence-Shell Electron-Pair Repulsion (VSEPR) model
of molecular structure assumes that areas of valence electron density around
an atom are distributed to be as far apart as possible in three-dimensional space.
- When using the VSEPR model, lone pairs of electrons, the two electrons
in a single bond, the four electrons in a double bond, and the six electrons
in a triple bond are each counted as only a single area of electron density.
- Four areas of electron density around an atom adopt a tetrahedral shape with
bond angles near 109.5°, such as in methane, CH4.
- Three areas of electron density around an atom adopt a trigonal planar shape
with bond angles near 120°, such as in formaldehyde, H2C=O.
- Two areas of electron density around an atom adopt a linear shape with bond
angles near 180°, such as in acetylene or CO2.
- The VSEPR model predicts shape but does not explain why the electrons are
located where they are. Thus, it is only a useful model
and not a theory.
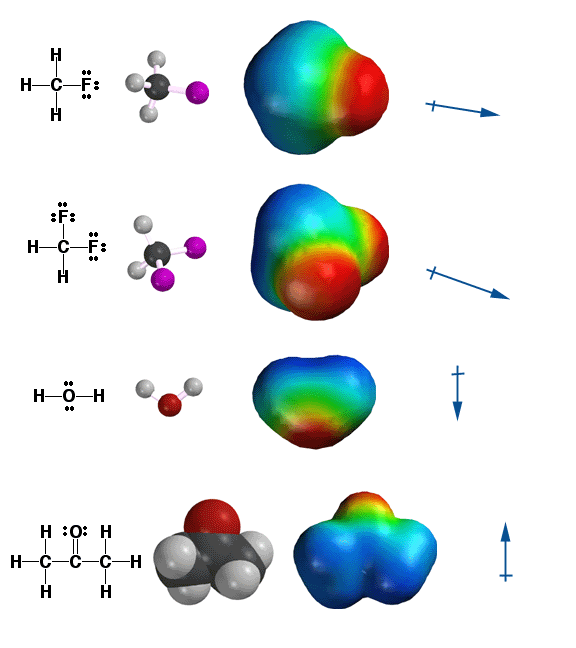
Shown
above are the Molecular Dipole Moments for some common molecules. Molecular
dipole moments are the vector sums of the individual bond dipole moments
in a molecule. Being able to predict in a qualitative way what a molecular
dipole moment looks like is important for this class because it is requires
the synthesis of all the ideas about electronegativity and molecular three-dimensional
shapes that we have discussed thus far.