SN2
Reaction: Backside attack of the nucleophile
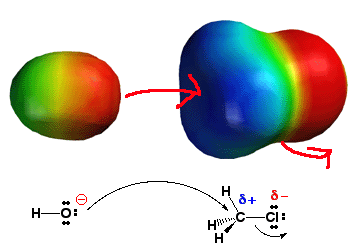
SN2
reactions are initiated through reaction of a nucleophile on the backside
of the electrophile carbon-halogen bond. The
charge distribution of the alkyl halide electrophile (where the electrons
are!) promotes this process as seen above. The nucleophile with a large
negative charge (red color) is attracted to the backside of the C-X bond
due to partial positive charge (blue color) present there. Thus, by understanding
electron distribution (where the electrons are!), you can correctly understand
and predict that the nucleophile will initiate the SN2
reaction via backside attack of the C-X bond. Click
here to see the SN2 mechanism movie.
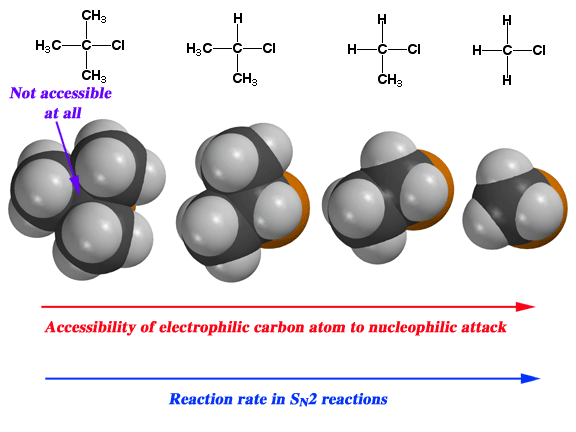
SN2
Reaction: Steric hindrance
Pictures of the Day CH320M/CH328M
11-05-25
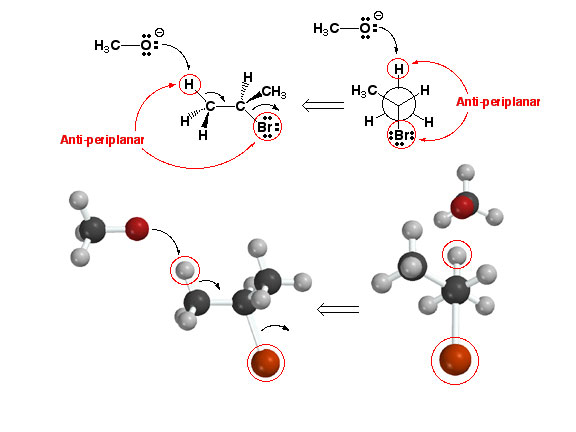
Anti-periplanar
Geometry and E2 Reaction
An
E2 reaction involves the deprotonation of a
C-H bond with simultaneous (concerted) formation of a new pi bond and
loss of a leaving group such as halide anion to give an alkene. The most
important thing to remember about E2 reactions is that an anti-periplanar
geometry is required for the reaction to take place, since
this geometry is the only one that properly aligns the orbitals involved
with the reaction. Click
here to see a movie of an E2 reaction.