Pictures of the Day CH320M/CH328M
11-10-25
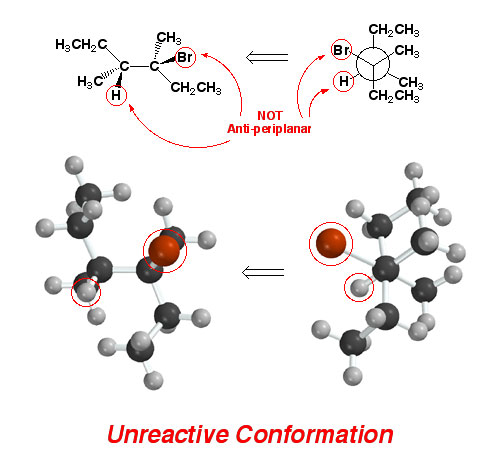
Sometimes
haloalkanes must undergo bond rotation before they are able to react by
the E2 reaction mechanism. Shown above is an alkyl halide in a conformation
that DOES NOT ALLOW an E2 process because
there is no hydrogen anti-periplanar to the halogen atom. Note there are
other hydrogen atoms in the molecule that could potentially react to some
small extent, but we are ignoring these because we are focusing on the reaction
that gives the predominant Zaitsev (most highly substituted) alkene product.
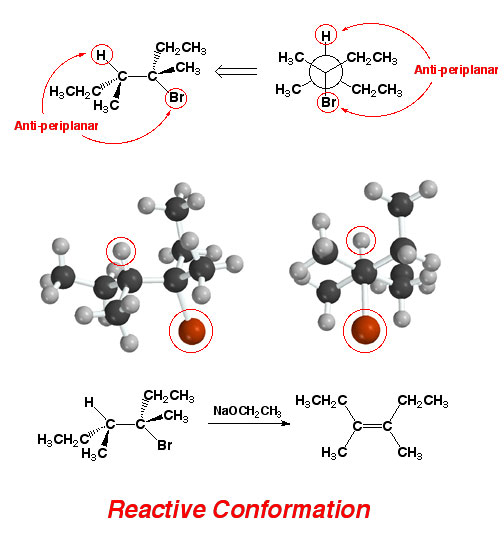
By
rotating the central C-C bond, a configuration can be found in which the H
and Br are anti-periplanar with respect to each other. This will be the conformation
that leads to reaction, meaning that the only product observed will be the Z alkene as shown. Thus, being able to identify the reactive conformation
(H and Br are anti-periplanar allows you to predict correctly the alkene product
(E vs. Z).
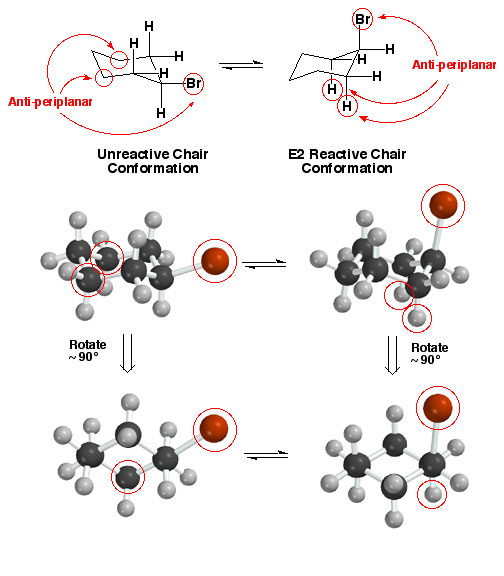
Cyclohexyl
Halides - Halide Must Be Axial
An
important consequence of the required anti-periplanar geometry for an E2
reaction is that only cyclohexyl derivatives with
the halogen atom axial can take part in the reaction. The reason,
as shown above left, is that when the halogen atom is equatorial, only the
carbon atoms circled are anti-periplanar with respect to the halogen atom.
There are no hydrogen atoms that can be removed according to the E2 mechanism!
The anti-periplanar atoms are the two circled carbon atoms. On the other
hand, when the ring flips and the halogen atom is axial, either of the two
circled axial hydrogen atoms can take part in an E2 reaction.