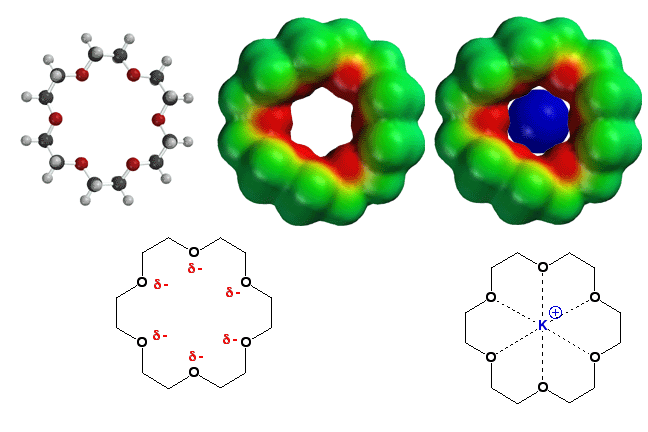
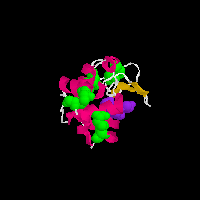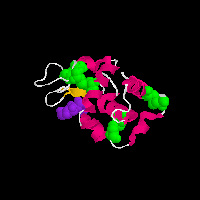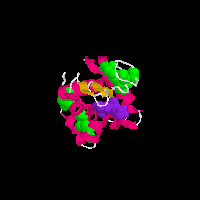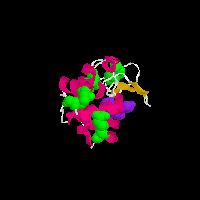Crown
Ethers - Selectively Bind Metal Ions
The
Importance of -S-S- Bonds in Biochemistry
Beta-lactamase
Lysozyme
Click
on the pictures and you will see two animated GIFs of proteins that contain
disulfide (-S-S-) bonds. In the above structures, the disulfide bonds are
shown in green as space filling models, the rest of the protein structure
is shown in a cartoon format in which the chain of the amino acids is shown
as a helix (pink), loop reagion (thin white) or beta sheet (burnt organe).
Both of these proteins also have a substrate analog bound to them (purple)
that is shown for reference. The grenn covalent disulfide bonds provide a
link between distant parts of the protein structure and thus serve to stablize
the three-dimensional folded structure of the protein.
Pictures of the Day CH320M/CH328M
11-17-25
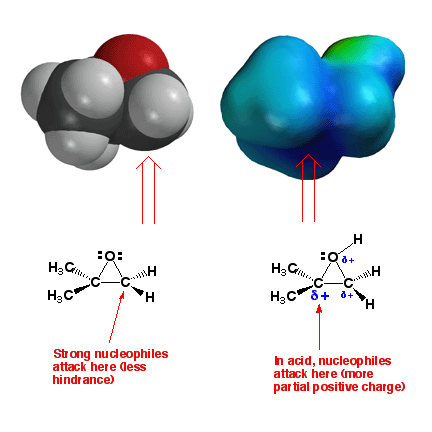
Epoxides
- Reactions with Nucleophiles
Epoxides react
with nucleophiles because this allows the opening of the three-membered
ring and relief of ring strain (thus providing motive). In an epoxide such
as the one shown above with different number of substituents on the carbons
of the ring, strong nucleophiles like HO- will react predominantly on the
backside of the less hindered carbon atom. In acid, a positively-charged
intermediate analogous to a bromonium or mercurinium ion is produced. Reaction
with nucleophiles such as H2O will occur at the more
substituted carbon atom, because this is the one with more partial postive
charge (blue color). Thus the regiochemistry can be controlled by choosing
whether to use acid. Note that the sterochemistry of addition is anti in
both cases (there is backside attack in both cases), leading to trans products.