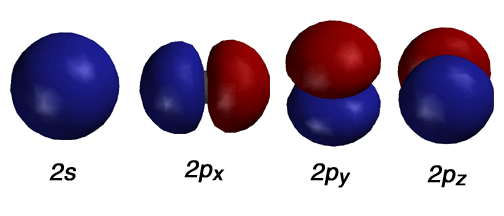9-3-25 (oroginally recorded 9-4-24)
Sigma
Bond (H2)
Bonding
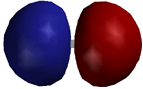
Sigma Molecular Orbital of the H2 Molecule
- A Sigma Bond that holds
two electrons.
The
sigma bonding molecular orbitals is shown
for the H2 molecule. You can tell this is a sigma bond
in that there is cylindrical symmetry around the bond axis. In the case of
H2 the sigma bonding interaction results from overlap
of the 1s orbitals on each H atom. Recall that a sigma bond is a bond in which
the majority of the electron density is between the two nuclei. A sigma bond also results from the overlap of an sp3, sp2 or sp
hybrid orbital and any s, sp3, sp2 or sp hybrid orbital. Because rotating
a sigma bond does not decrease the overlap of the orbitals involved (sigma
bonds have cylindrical symmetry), a sigma bond can rotate freely about the
bond axis.
Pi
Bonds
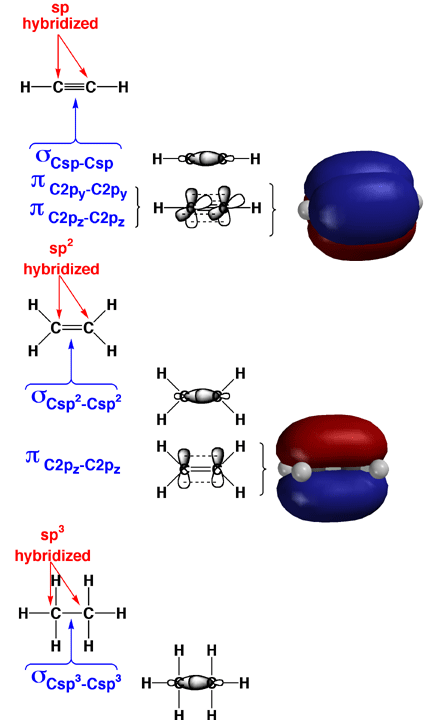
Above
is the structure of ethylene, acetylene and ethane, including the representations of the pi bonds.
The two carbons of ethylene are joined by a double bond and share four electrons.
Two electrons are located in the sigma bond fromed from the overlap to two sp2 hybrid orbitals and the electron density is located
between the two carbons. The remaining two electrons exist in the pi bond
that is formed from the overlap of the 2p orbitals on each sp2 hybidized
carbon atom. For acetylene, the two carbons of acetylene share 6 electrons and are joined by a triple bond. Two electrons are located in the sigma bond formed from the overlap of sp hybrid orbitals and the electron density is located
between the two carbons. The remaining four electrons exist in the two orthogonal pi bonds
that are formed from the overlap of the 2p orbitals on each sp hybidized
carbon atom. Ethane on the bottom has two carbons joined by a single bond and share 2 electrons. The two electrons on located in the sigma bond formed by overlap of two sp3 hybrid orbitals.
Pictured above are the atomic orbitals most interesting to
an organic chemist. In particular, the 2s, and three 2p orbitals are displayed.
These are interesting because they hold the valence electrons for atoms
such as C, N, O and F. Here, the red and blue correspond to areas of positive
and negative amplitude of the wave function. THE SIGN OF THE AMPLITUDE
OF THE WAVE FUNCTION HAS NOTHING TO DO WITH CHARGE. You are just as likely
to find electron density in a lobe with positive amplitude (+) as it is
to find it in a similar lobe with negative amplitude (-). Recall that the
wave function is the particular solution to the Schrodinger equation that
describes each orbital.
BOTTOM LINE: READ THIS IF ORBITALS FRIGHTEN YOU:
This material is very complex, yet what you should know at this point is rather
simple:
1. Electrons have properties of waves (you should know what those properties
are)
2. The wave functions help us understand the location of electron density around
an atom.
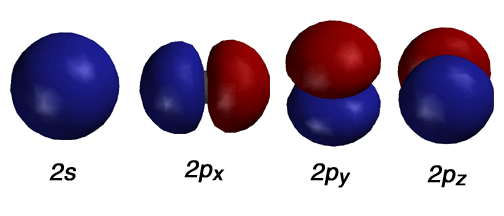
Sigma
Bond (H2)(One more time)
Antibonding
Sigma Molecular Orbital of the H2 Molecule-The
concept of an antibonding sigma orbital is rarely used in organic chemistry
other than the notion that filling an antibonding orbital with two electrons
amounts to breaking that sigma bond.
Bonding
Sigma Molecular Orbital of the H2 Molecule
- A Sigma Bond that holds
two electrons.
The
sigma bonding (below) and antibonding (above) molecular orbitals are shown
for the H2 molecule. You can tell this is a sigma bond
in that there is cylindrical symmetry around the bond axis. In the case of
H2 the sigma bonding interaction results from overlap
of the 1s orbitals on each H atom. In the case of the antibonding orbital
shown above, the red and blue correspond to areas of positive and negative
amplitude of the wave function. Recall that a sigma bond is a bond in which
the majority of the electron density is between the two nuclei. A sigma bond
can result from the overlap between an s orbital and any other type of atomic
orbital. A sigma bond also results from the overlap of an sp3, sp2 or sp
hybrid orbital and any s, sp3, sp2 or sp hybrid orbital. Because rotating
a sigma bond does not decrease the overlap of the orbitals involved (sigma
bonds have cylindrical symmetry), a sigma bond can rotate freely about the
bond axis. Note that the sigma antibonding orbital is not filled with any
electron density under normal circumstances. Thus, the main
take home message from this graphic is what a sigma bond looks like, namely
the bonding molecular orbital shown on the bottom.
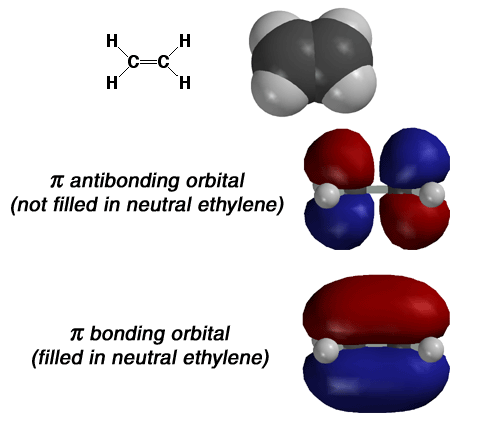
Above
is the structure of ethylene, including the representations of the pi bonds.
The two carbons of ethylene are joined by a double bond and share four electrons.
Two electrons are located in the sigma bond and the electron density is located
between the two carbons. The remaining two electrons exist in the pi bond
that is formed from the overlap of the 2p orbitals on each sp2 hybidized
carbon atom. The interaction of the 2p orbitals in a pi bond create one bonding
molecular orbital and one antibonding molecular orbital. In a neutral state,
the electrons in the 2p orbitals of ethylene will combine in phase, in the
bonding molecular orbital. This occurs because the electrons in the pi bond
will be in the lowest energy state and thus give the molecule the most stability.
It is also important to realize that another molecular orbital exists. This
is called the pi antibonding molecular orbitals and is shown above the pi
bonding orbital. Remember, this orbital does exist but no electrons are present
in this orbital if the molecule is neutral. Electrons move into the antibonding
orbital only when they are excited, say by UV light, and thus attain a higher
energy state. Also, note that the antibonding molecular orbital has a node
between the two nuclei. Again, I have introduced the concept of antibonding
orbital only for the sake of completeness. The main take home message
from this graphic is what a pi bond looks like, namely the bonding pi molecular
orbital shown on the bottom.